Image credit: Canva
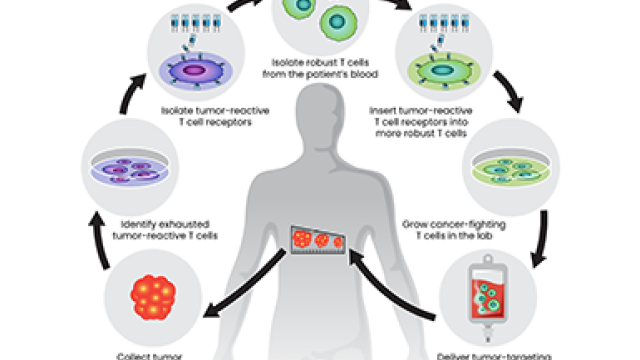
Colorectal cancer that has returned or progressed after treatment and spread to other organs is known as metastatic colorectal cancer (mCRC). Most people with mCRC survive only about two years. A trial led by Jason M. Redman, M.D., Assistant Research Physician in the Center for Immuno-Oncology, is studying combination immunotherapy, including a tumor-targeted vaccine, for the disease. The trial includes two mandatory biopsies.
The trial will take place at the NIH Clinical Center in Bethesda, Maryland, and there is no cost for participation.
For more information, please contact the NCI Medical Oncology Referral Office at (888) 624-1937 or ncimo_referrals@nih.gov.
Clinicaltrials.gov identifier: NCT06149481
NCI Protocol ID: IRB001563
Official Title: Phase I/II Study of the Combination Immunotherapy Regimen: SX-682, TriAdeno Vaccine, Retifanlimab and IL-15 Agonist N-803 (STAR15) for Metastatic Colorectal Cancer (mCRC)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow, treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.