Zhengping Zhuang, M.D., Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37, Room 1000
- Bethesda, MD 20892
- 240-760-7055
- 240-541-4465
- zhengping.zhuang@nih.gov
RESEARCH SUMMARY
Dr. Zhuang is a research scientist who lead the Cancer Stem Cell Biology Research Program in the Neuro-Oncology Branch (NOB). His program used critical patient observations to drive bench research, which is then translated for clinical use. He has investigated the role of hypoxia signaling in tumor pathophysiology throughout his career, which led him to discover a unique neuroendocrine tumor syndrome caused by a somatic gain-of-function mutation in the genes encoding hypoxia-inducible factor 2α (HIF2α), causing Pacak-Zhuang syndrome. This model helped him explore the role of hypoxia signaling in tumor pathophysiology, including brain tumors.
Areas of Expertise
Research
The root of Dr. Zhuang’s research was based on the inherited and somatic mutations that result in cancer. He aimed to understand how these mutations contribute to the development and progression of tumors, particularly in brain tumors. His Cancer Stem Cell Biology Research Program at the Neuro-Oncology Branch (NOB) utilized clinical, translational, and basic approaches to study the basic science as a foundation for understanding the pathological changes present in tumors. He had three ongoing multidisciplinary projects: 1) investigating hypoxia signaling and tumor development, 2) examining the impact of patient-derived IDH1 mutant mosaicism on brain tumor development, and 3) developing therapeutics for cancers of the central nervous system.
The first project investigated gain-of-function mutations in the gene encoding the hypoxia inducible factor 2α (HIF2α) protein, which was first found in a combined clinical and scientific effort culminating in the discovery of Pacak-Zhuang syndrome. Using mouse models, Dr. Zhuang’s lab investigated the unusual phenotypes recently identified in patients with Pacak-Zhuang syndrome, including sexual dimorphism and HIF2α genomic imprinting. These studies will substantially advance our understanding of HIF2α’s impact on biology and pathobiology, including Pacak-Zhuang syndrome and hypoxia signaling in brain tumors.
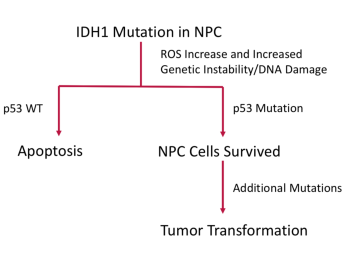
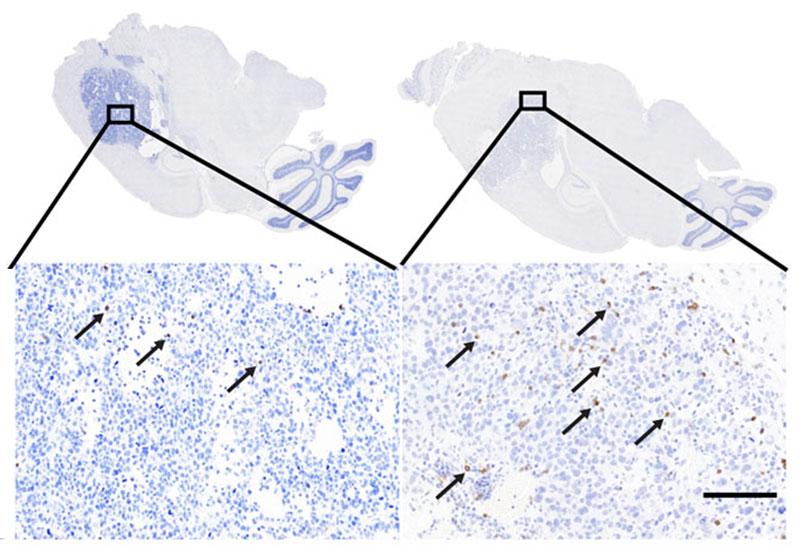
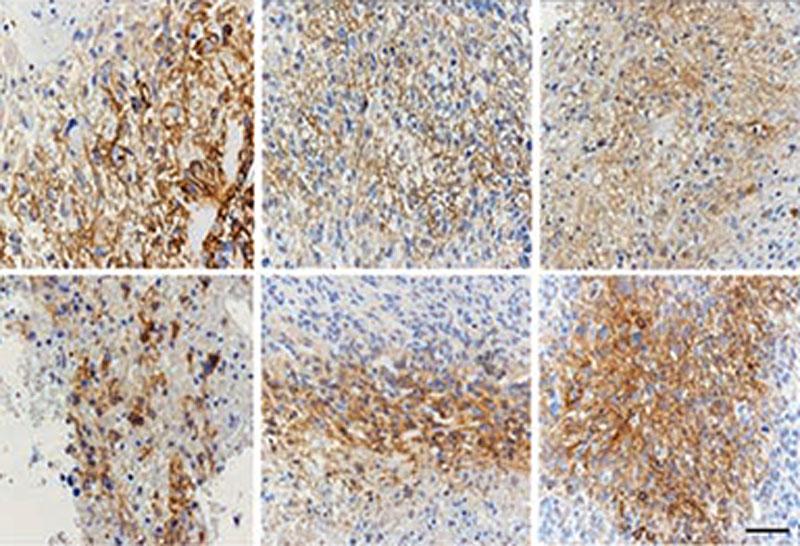
The second project began with a critical observation in identical twins with low-grade IDH-mutant gliomas. This observation showed that an IDH1 mutation in early development can have two possible outcomes: 1) it may remain quiescent or in the “pre-tumor state” throughout development, or 2) it may acquire additional mutations that allow the cell to transform into a tumor. Because Dr. Zhuang discovered glutamine synthetase, which is important in IDH-mutant tumors (and astrocytoma specifically), his lab was primed to deduce the basic physiology resulting in a brain tumor. His team used deep PCR techniques to show that an inherent and embryonic IDH1 mutation triggered a tumor with p53. His lab also used induced pluripotent stem (iPS) cells to determine how IDH mutations drive tumor growth by studying the 2-hydroxyglutarate (2-HG) and glutamate cycle.
The third project had several arms: First, Dr. Zhuang’s lab worked to develop an immunomodulator called LB100, which acts by inhibiting protein phosphatase 2A and has entered clinical trials. Second, his lab developed CAR-T cells targeting the hypoxia signaling downstream product, carbonic anhydrase IX. Third, he developed a modulator of HIF2a, which allowed his lab to further explore this pathway.
Publications
Transgenic Mouse Model of Polycythemia, Paraganglioma, and Somatostatinoma with Epas1 Gain-of-function Mutation.
Targeting Hypoxia Downstream Signaling Protein, CAIX, for CAR-T Cell Therapy against Glioblastoma.
Pharmacologic inhibition of protein photastase-2A, with novel inhibitor LB-100, achieves durable immune-mediated antitumor activity when combined with PD-1 blockade.
β-catenin signaling initiates the activation of astrocytes and its dysregulation contributes to the pathogenesis of astrocytomas
Somatic HIF2A gain-of-function mutations in paraganglioma with polycythemia
Biography

Zhengping Zhuang, M.D., Ph.D.
Dr. Zhuang received his Doctor of Medicine degree from Shanghai Jiao Tong University School of Medicine (formerly Shanghai Second Medical University) in China. He completed a short residency in general surgery at Ruijin Hospital before moving to the United States to pursue a Ph.D. in pharmacology at Wayne State University in Detroit, Michigan. Following his doctorate, he was a postdoctoral fellow at Massachusetts General Hospital for a year. Shortly thereafter, he relocated back to Michigan to be a resident in translational medicine at Henry Ford Hospital. He then served as a research associate at the University of Michigan, and moved to NIH in 1993 as a resident in anatomic physiology at NCI’s Department of Pathology. He was named a special expert and staff pathologist for the Surgical Neurology Branch—a position he held for seventeen years. In 2017, Dr. Zhuang joined the Neuro-Oncology Branch (NOB) as a senior investigator and head of the Cancer Stem Cell Biology Research Program.
In addition to his extensive research experience and tenure in multiple branches at NIH, Dr. Zhuang is also licensed to practice medicine in the District of Columbia, and holds a completion certificate for an anatomic pathology residency. He also currently holds five patents (11 pending) that highlight the novelty of his research endeavors.
Dr. Zhuang retired from CCR in June 2025.
Honors, Awards and Leadership
- The Federal Technology Transfer Award, 2019
- The Federal Technology Transfer Award, 2018
- Cy Katzen Humanitarian Award, 2014
- Federal Laboratory Consortium Award for Excellence in Technology Transfer, 2010
- NIH APAO Outstanding Achievement Award, 1999
- Gordon F. Vawter Award, Society for Pediatric Pathology, 1996
- NCI New Invention and Technology Development Award, 1995
- American Society of Clinical Pathologists Resident Award in Anatomic Pathology, 1994
- Stowell-Orbison Award for Pathologist-in-training, U.S. and Canadian Academy of Pathology, 1994
Patents
- Isolation of cellular material under microscopic visualization using an adhesive/extraction reagent tipped probe, US Patent #5843644
- Isolation of cellular material under microscopic visualization, US Patent #6010888
- Isolation of cellular material under microscopic visualization, US Patent #6204030
- Isolation of cellular material under microscopic visualization, US Patent #6569639
- The gene associated with Multiple Endocrine Neoplasia type 1, menin polypeptides, and uses thereof, US Patent # 7358347
News
A New Vaccine to Target Treatment-Resistant Glioblastoma
March 8, 2024
The rWTC-MBTA vaccine extended survival and prevented tumor recurrence in preclinical brain tumor models. Read more >
Examining How Brain Tumors Thrive in Low Oxygen Environments to Develop Targeted Therapies
March 3, 2023
Dr. Zhengping Zhuang combines clinical observations and preclinical models to understand how glioblastoma cells multiply rapidly in oxygen-depleted environments during tumor development. Read more >