Researchers conducted preclinical experiments to explore a treatment that damages brain tumors’ DNA and prevents the cells from repairing it.
Raleigh McElvery, Scientific Communications Editor
September 19, 2023
Glioblastomas are the most common primary brain cancer in adults. They are fast-growing and often treatment resistant. As a result, current therapies usually only extend patients’ lives by a few months. To improve treatment options, researchers at the NCI Center for Cancer Research’s Neuro-Oncology Branch (NOB) are investigating more precise, personalized therapies that take into account each tumor’s genetic makeup.
While no two glioblastomas are the same, roughly 40% share a common characteristic: They are deficient in an enzyme known as phosphatase and tensin homolog (PTEN). Encoded by the PTEN gene and commonly referred to as a tumor suppressor, PTEN stops cells from multiplying uncontrollably. However, cancer cells with less PTEN (or no PTEN) have no such control. They grow uninhibited, often leading to a worse prognosis.
Although PTEN deficiency allows glioblastomas to grow and spread, this genetic alteration may create a weakness that makes them more sensitive to treatment. That’s because, in addition to suppressing cell growth, PTEN also helps cells repair their DNA after it’s been damaged. As a result, PTEN-deficient glioblastoma cells have a harder time fixing their broken DNA—which can be a fatal flaw.
In a new study published in Neuro-Oncology Advances, researchers in the NOB’s Translational Research Program took advantage of this vulnerability. By conducting preclinical experiments, they identified a drug combination that induces DNA damage and prevents PTEN-deficient glioblastoma cells from repairing it, thus killing the tumor cells. The researchers also determined that their drug combination could cross the blood-brain-barrier that protects the central nervous system (CNS), suggesting that the treatment will be able to reach and target brain tumors in people.
“There’s no treatment approach that will be effective for every glioblastoma,” explains Jing Wu, M.D., Ph.D., head of the NOB’s Translational Research Program and the study’s senior author. "We are hoping the findings from our study can lead to a therapy for a subset of glioblastoma patients whose tumors harbor this particular tumor-driving genetic alteration.”
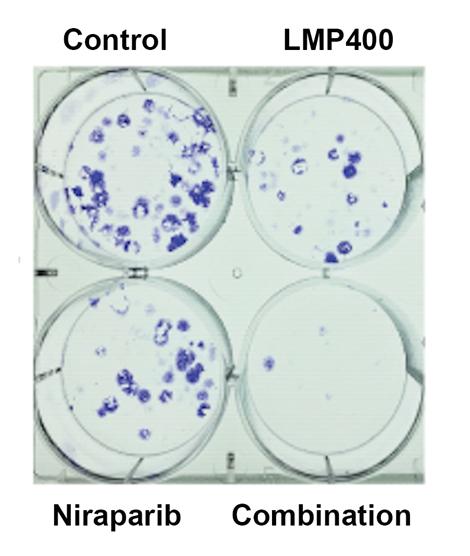
The two drugs that the researchers combined are called LMP400 (Indotecan) and Niraparib, respectively. LMP400 was discovered by coauthor Yves Pommier, M.D., Ph.D., in 2007 and is known as a topoisomerase I inhibitor—a drug that interrupts cell division and causes DNA damage. Niraparib is known as a PARP inhibitor, which prevents cells from repairing their damaged DNA, eventually leading to cell death. PTEN-deficient glioblastoma cells already have a hard time repairing damaged DNA, so the mix of LMP400 and Niraparib proved crippling.
Although topoisomerase I inhibitors and PARP inhibitors have been combined before to treat solid tumors and lymphoma, the new study is the first to test this dual approach in brain tumors. Niraparib is FDA-approved to treat advanced epithelial ovarian, fallopian tube, and primary peritoneal cancers, and is currently being tested in clinical trials to target brain tumors. LMP400 has not been tested in brain tumors, but the NOB researchers suspected it would be effective against glioblastomas because it is extremely potent and has fewer side effects than other topoisomerase I inhibitors.
“This is a very special drug combination,” says Olga Kim, M.D., Ph.D., an NOB postdoctoral fellow and the study’s first author. “Not only can LMP400 and Niraparib reach the brain to treat tumors there, but they are able to target this very specific subset of glioblastomas. Glioblastoma patients do not have many treatment options, and we hope that this precision medicine approach will help them receive better care.”
Through a series of experiments conducted in cell lines and mice, the researchers found that PTEN-deficient glioblastoma cells are particularly sensitive to LMP400. Adding Niraparib augmented these effects and killed more tumor cells, in addition to prolonging survival in a mouse model.
“Oftentimes glioblastomas will develop resistance to a single drug agent, so combining the two drugs can help overcome this resistance,” Dr. Kim says. “It also means we can use a lower dose of each drug, which decreases the likelihood of side effects.”
In addition to demonstrating the synergistic effects of using the two drugs in combination, the researchers also investigated whether LMP400 and Niraparib could pass the blood-brain-barrier. The blood-brain-barrier contains proteins called ABC transporters, which protect the CNS by sensing foreign substances and pumping them back out into the bloodstream. The researchers were pleased to see that neither drug was affected by the transporters—unlike other topoisomerase I inhibitors and a common PARP inhibitor that the team tested called Olaparib. This signified that the drugs would likely be able to reach brain tumors in patients.
According to Dr. Wu, the fact that PTEN deficiency sensitizes glioblastoma cells to this combined therapeutic approach is extremely exciting. She hopes their results will allow them to help people with this type of glioblastoma.
“This study sets a strong scientific foundation that could help to meet the unmet needs of glioblastoma patients,” she says. In the future, her team plans to translate these preclinical findings to clinical trials to test the benefit to people with glioblastoma—and ultimately improve their care and outcomes.