A technique called Raman spectroscopy has revolutionized the way Dr. Mioara Larion’s lab understands tumor cell metabolism—and may ultimately help researchers design more effective cancer drugs.
By Raleigh McElvery, Scientific Communications Editor
November 28, 2022
In the lab of Mioara Larion, Ph.D., researchers are peering deep inside cancer cells to expose how tumors redirect their energy in order to grow unchecked. Each member of Dr. Larion’s lab specializes in a different method to examine the set of life-sustaining chemical reactions—broadly known as metabolism—that fuels tumor cell growth and division. Several years ago, one such technique, called Raman spectroscopy, spurred a discovery that changed the course of Dr. Larion’s research trajectory.
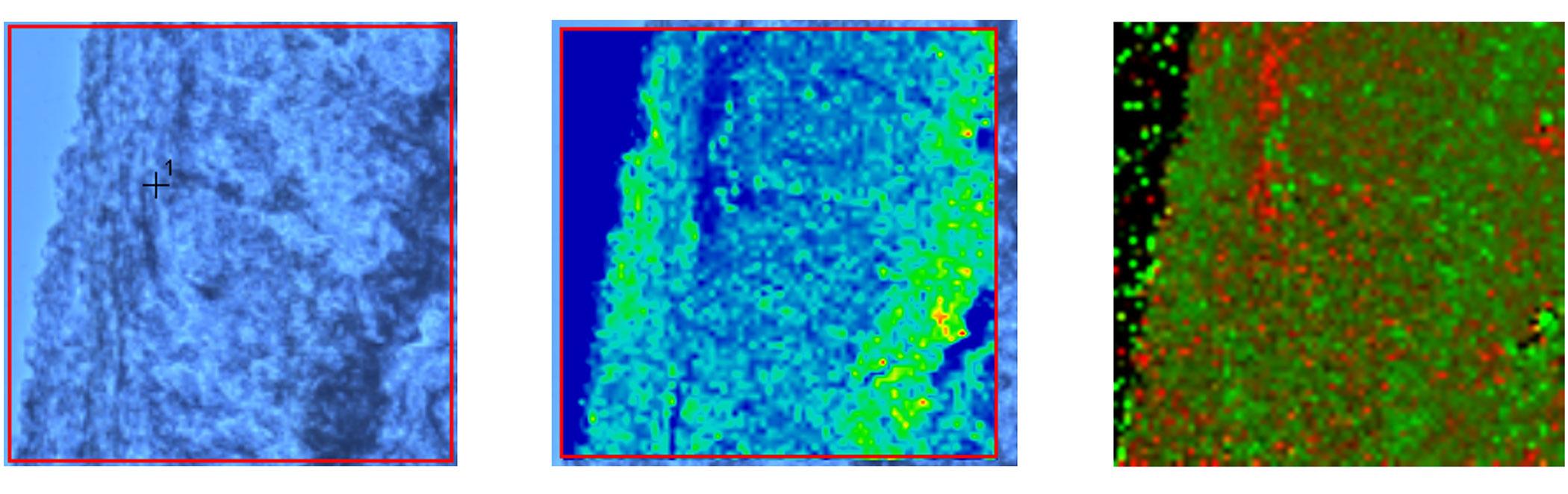
Many labs employ Raman spectroscopy, but only a small subset—including Dr. Larion’s Cancer Metabolism Research Program—use it to examine cancers of the brain and spine. A spectrometer works by quite literally shining a light on these rare tumors. It shoots a powerful laser at the tissue sample and the light ricochets off the cells, scattering at different wavelengths depending on the molecules inside the cell and the way the chemical bonds in those molecules vibrate. Dr. Larion’s team can then use this information to deduce which byproducts of metabolism are present, as well as their concentrations. By piecing together the chemical reactions taking place, Dr. Larion aims to understand how cancer cells rewire their metabolism to grow uncontrollably and form tumors.
She is particularly interested in the sets of reactions that allow cells to store and break down fatty acids known as lipids, which form membranes that protect the cell and its many compartments (also called organelles), including the mitochondria, endoplasmic reticulum, Golgi apparatus, and others. Understanding lipid metabolism, she says, will ultimately help researchers design more effective and less toxic cancer drugs, in addition to combatting drug resistance and determining the best ways to combine treatment approaches.
“The brain is very rich in lipids, and we think there is something about this environment that makes cancer cells especially susceptible to altered lipid metabolism,” she explains. “Our goal is to create a Raman spectroscopy-based toolbox that is specifically tailored to these cancers, in order to accurately measure metabolic reactions in live cells and tissues.”
A Laser Focus on Lipid Metabolism
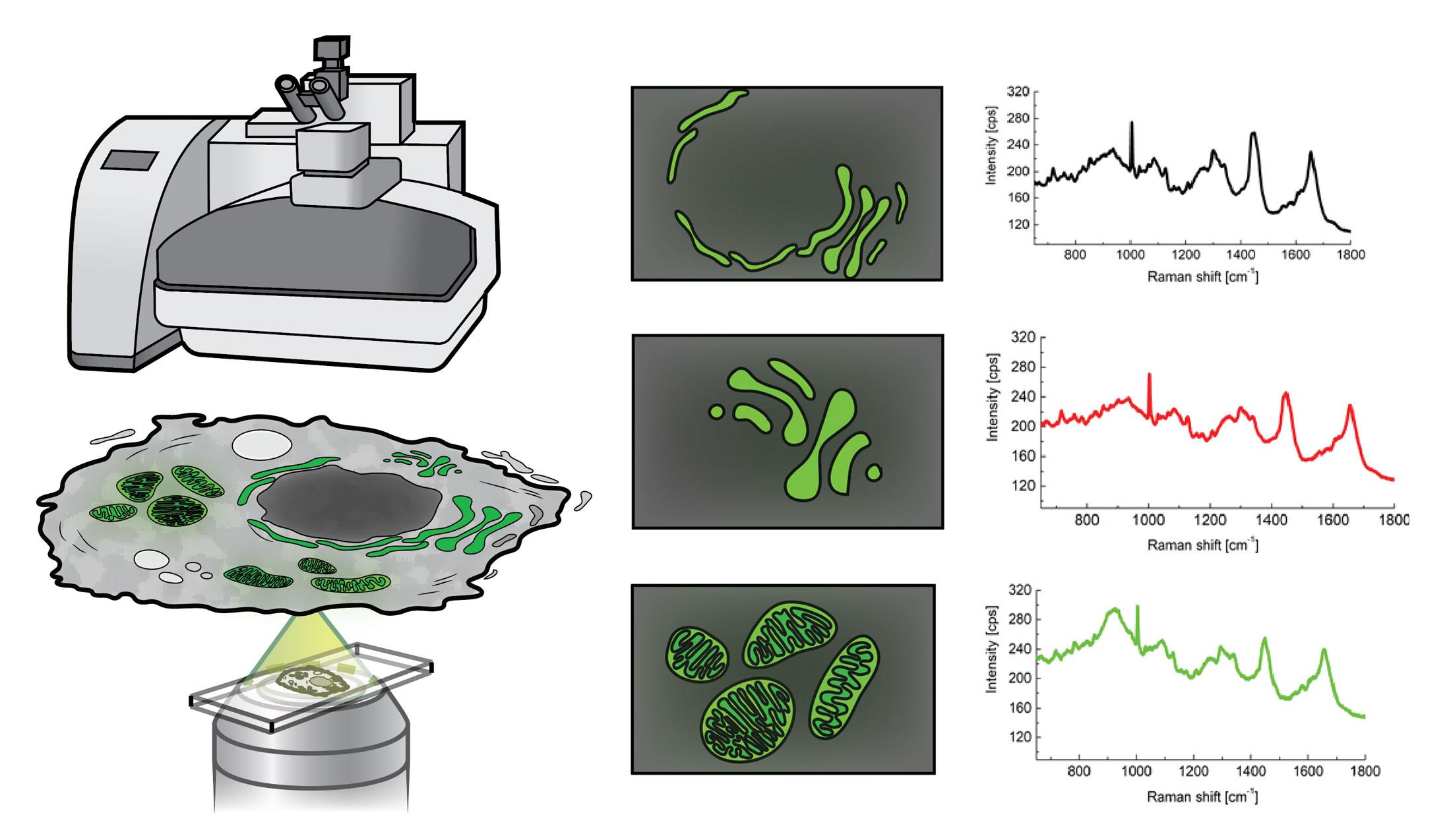
Traditionally, scientists could only glean a bird’s eye view of all the metabolic reactions occurring in all the compartments in a cell, across millions of cells in a tissue. However, this information is so general that it’s nearly impossible to disentangle what metabolic reactions are happening in which parts of the cell. To address this issue, Dr. Larion’s team has taken a more granular approach: By coupling a Raman spectrometer to a fluorescent microscope, they can pinpoint where to aim the spectrometer’s laser to separate the metabolic reactions taking place in specific cellular compartments.
Dr. Larion compares understanding how metabolism powers a cell to examining the parts of a car. “You can’t just open the hood and say, ‘Oh this is what’s gone wrong, let’s fix it.’ You have to analyze each component under the hood in order to find the problem.”
While other methods for examining subcellular lipid metabolism require scientists to remove a cell’s individual compartments before analyzing them, Raman spectroscopy is non-invasive and can be performed on live, intact tissue. It also allows researchers to drill down to the scale hundreds of times smaller than a human hair. This provides a detailed picture of what’s going on inside a cell’s miniscule compartments, but also generates huge amounts of complex data in the process. To help interpret this convoluted data, researchers like Dr. Larion are devising “deconvolutional” algorithms that make it easier to determine which metabolic products (such as lipids) are present, as well as how they are structured.
Research Fellow Adrian Lita, Ph.D., has been a member of Dr. Larion’s group for six years and played an important role in designing this algorithm, called BCAbox. As the lab’s resident Raman spectroscopy expert, Dr. Lita has extensive training in physics and chemistry, and has employed Raman spectroscopy throughout much of his career. However, he had mostly used it to characterize inorganic materials, such as nanoparticles and salt, which have limited chemical components and a very clear, organized structure that is easy to recognize using a spectrometer. Cells, by comparison, are much more complex. A single subcellular compartment might contain hundreds or thousands of different chemicals, each of which scatters the light from the laser differently.
When Dr. Lita first joined Dr. Larion’s Cancer Metabolism Research Program in 2016, the lab was primarily focused on how cancer cells metabolize sugars and amino acids. But in 2021 all that changed. Together with their colleagues, Drs. Lita and Larion were examining a common type of brain tumor called an IDH1-mutant glioma, which harbors a genetic mutation that causes a cell’s metabolism to go haywire. Using Raman spectroscopy and their BCAbox algorithm, the researchers discovered that these tumor cells had a mysterious defect—some of their compartments contained a bizarre build-up of lipids, making them swell. As Drs. Larion and Lita explored their results further, they determined this strange lipid distribution had a clear cause: The genetic mutation in IDH1-mutant gliomas precipitated a cascade of very specific molecular events that ultimately created the lipid buildup.
This discovery was a turning point for the lab, Dr. Larion explains. “Raman spectroscopy has really refocused my group and motivated us to investigate lipid metabolism. It’s changed everything for us. I’m very excited about this new direction.”
A New Frontier of Raman Spectroscopy
In the years since this pivotal discovery, Dr. Larion’s team has started testing drugs that could target various steps of the molecular cascade leading to this lipid build-up, in hopes of curbing glioma growth. Thanks to generous support from philanthropists Ashley and Alan Dabbiere—who are avid brain tumor research supporters due to Ashley’s low-grade glioma diagnosis—the lab acquired a new Raman spectrometer in the summer of 2022: the state-of-the-art Leica STELLARIS 8.
“Our lab is just one of a few in the entire United States with access to this kind of instrument,” Dr. Lita says. “It’s incredibly exciting to have the capability to perform experiments that we wouldn’t have even dreamed of a decade ago.”
Most recently, the lab began collaborating with researchers from the University of Turku in Finland to create novel machine learning algorithms for analyzing the complex data generated by their Raman spectroscopy. These deep learning algorithms can quickly sift through Raman spectroscopy data, classifying gliomas based on their lipid composition and concentration, as well as other properties such as methylation status. While others have combined machine learning and Raman spectroscopy to differentiate tumor types from one another, Dr. Larion, Dr. Lita, and their collaborators are digging deeper to determine key biological differences between brain tumors and why those differences exist. The algorithm can also precisely delineate the margins of the tumor, showing surgeons which tissue is healthy and which is cancerous.
Raman spectroscopy is emerging as a critical tool to help doctors characterize tumors in a non-destructive manner—including in real time inside the operating room, says Ion Petre, Ph.D., an expert in computational biomodelling and Dr. Larion’s collaborator at the University of Turku. Combining Raman spectroscopy with machine learning will help unleash its full potential, Dr. Petre explains, and in turn provide mathematicians like him with rich data to feed their algorithms. His team is currently applying machine learning-based Raman spectroscopy to understand how tumors develop and evolve during treatment. This could help identify personalized treatment strategies for cancer patients, as well as prevent tumors from becoming drug resistant.
“Interdisciplinary work has great potential for expanding the frontier of science,” Dr. Petre says, “but it takes courageous and broad-minded scientists to develop such work. Dr. Larion is certainly one.”
As they continue to build their Raman spectroscopy toolkit, Dr. Larion’s lab has no shortage of exciting research avenues to pursue. In the future, she envisions using Raman spectroscopy and other methods to chart a patient’s own “metabolic map” that outlines their cells’ unique nutrient availability, genetic mutations, and metabolomic dependency—possibly even segregated by cellular compartment. This could help develop treatments tailored to a patient’s specific tumor.
As for Dr. Lita, each day when he arrives at his spectrometer, he’s motivated by the prospect of what he’ll discover next. “I’m learning new things all the time,” he says. “Cancer is a devastating disease, so we want to do everything we can to investigate tumor biology and accelerate advancements in diagnosis and treatment.”
Top image caption: Three thumbnails of normal brain tissue taken with an optical microscope (left) and by a Raman spectrometer coupled to a fluorescent microscope (middle and right). The middle Raman image shows the tissue's lipid distribution, while the right Raman image differentiates grey and white matter. Credit: Courtesy of the researchers