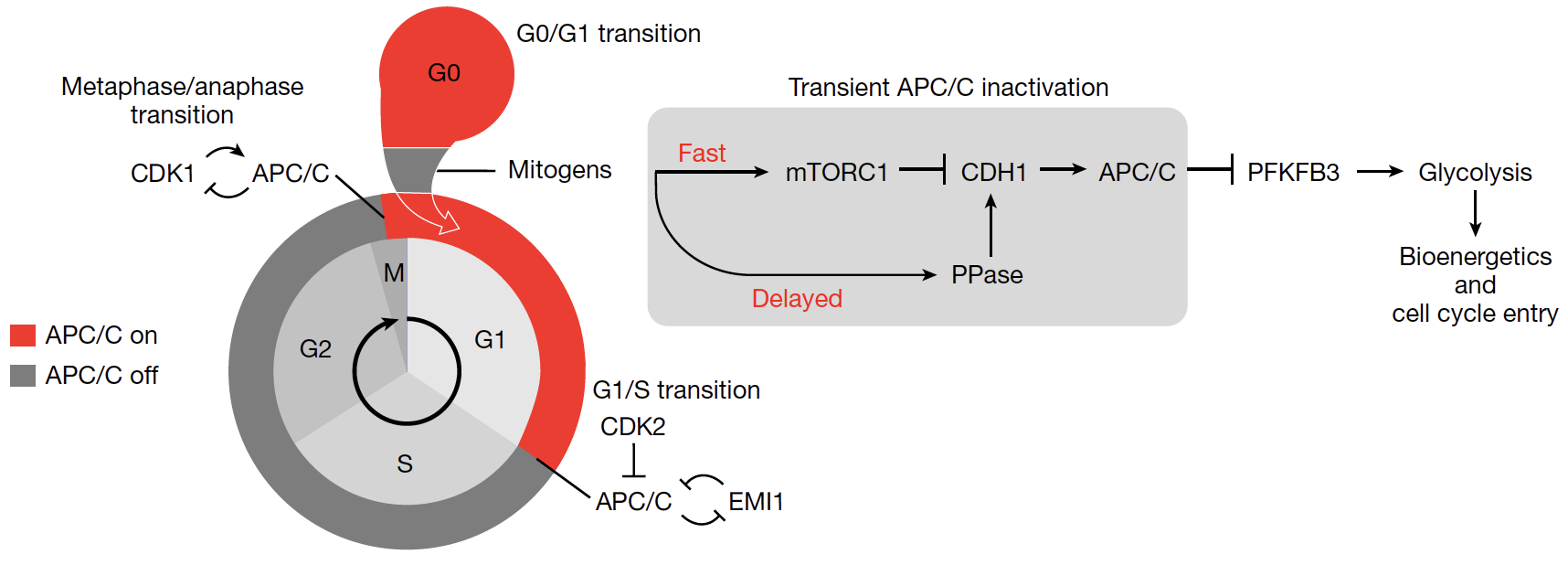
Transient APC/C inactivation by mTOR boosts glycolysis during cell cycle entry.
Paul D, Bolhuis DL, Yan H, Das S, Xu X, Abbate CC, Jenkins LMM, Emanuele MJ, Andresson T, Huang J, Albeck JG, Brown NG, Cappell SD. 2025.
Nature. 2025 July 30. doi: 10.1038/s41586-025-09328-w
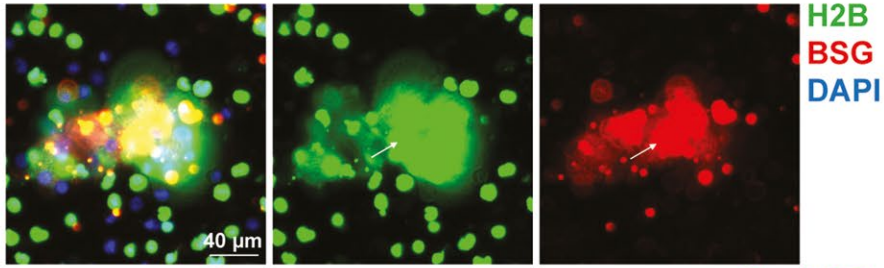
Proteomic analysis of tumor cell nuclear expulsion reveals significant cell adhesion and RNA binding programs in extracellular chromatin.
Gray JM, Park WY, Holewinski RJ, Andresson T, Carmona-Rivera C, Kaplan MJ, Yang L.
Scientific Reports. 2025 Aug 01;15:28054. doi: 10.1038/s41598-025-11807-z
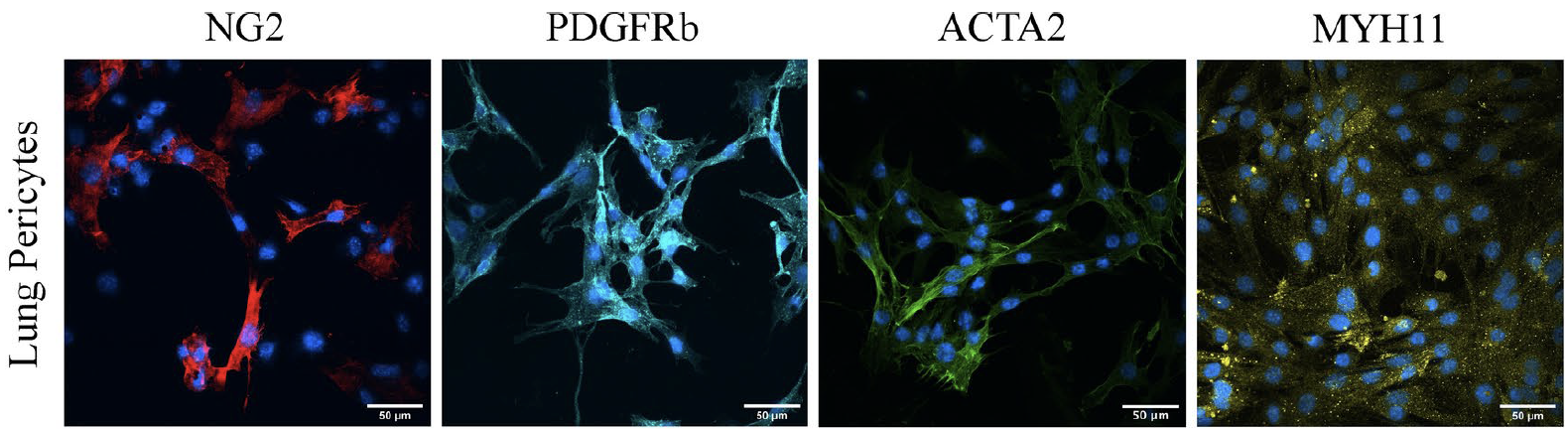
Pericytes require physiological oxygen tension to maintain phenotypic fidelity.
McErlain T, McCulla EC, Glass MJ, Ziemer LE, Branco CM, Murgai M.
Scientific Reports. 2024 Nov 28;14:29581. doi: 10.1038/s41598-024-80682-x
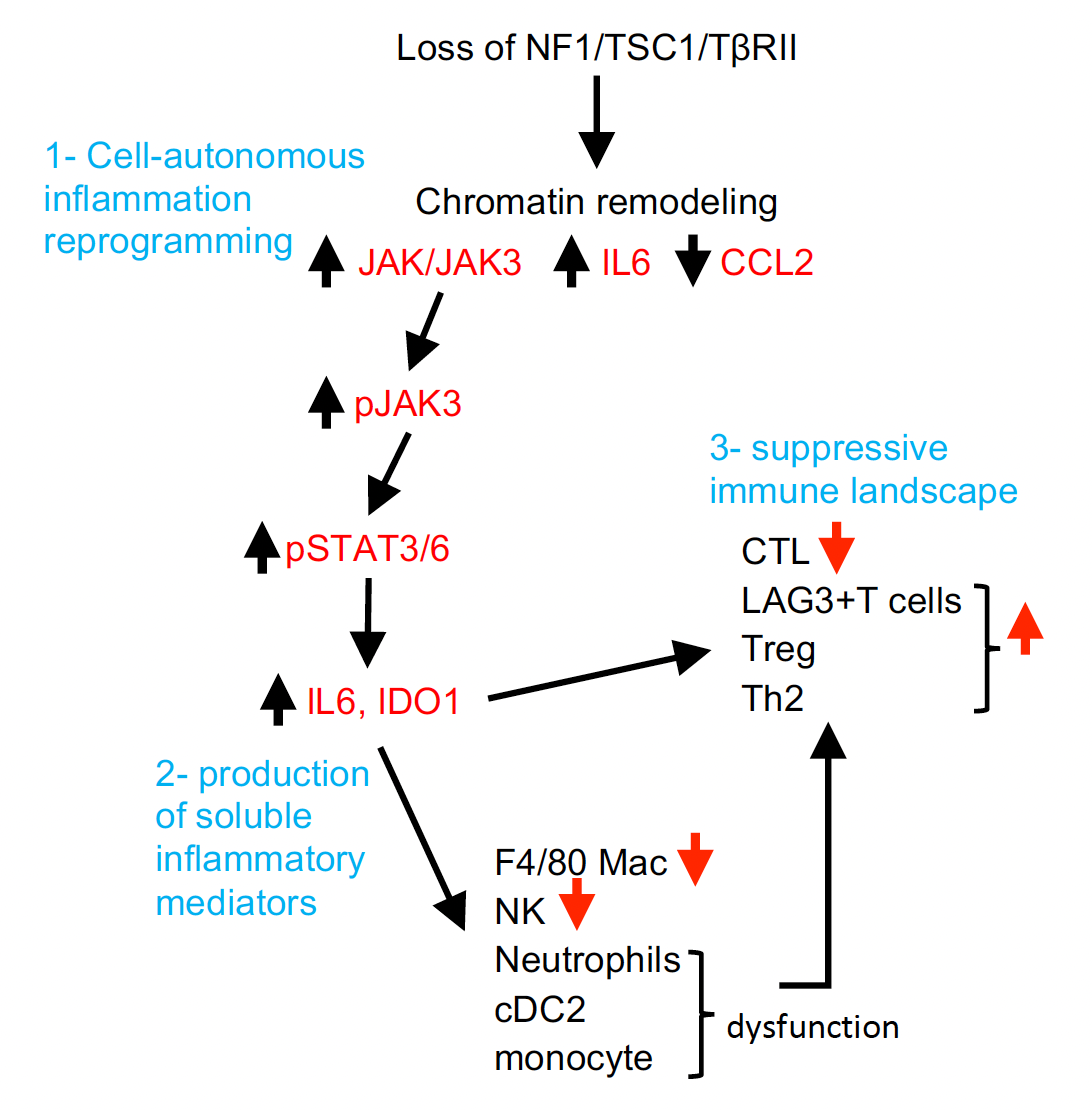
Loss of tumor suppressors promotes inflammatory tumor microenvironment and enhances LAG3+T cell mediated immune suppression.
Zahraeifard S, Xiao Z, So JY, Ahad A, Montoya S, Park WY, Sornapudi T, Andohkow T, Read A, Kedei N, Koparde V, Yang H, Lee M, Wong N, Cam M, Wang K, Ruppin E, Luo J, Hollander C, Yang L.
Nature Communications. 2024 July;15:5873. doi: 10.1038/s41467-024-50262-8
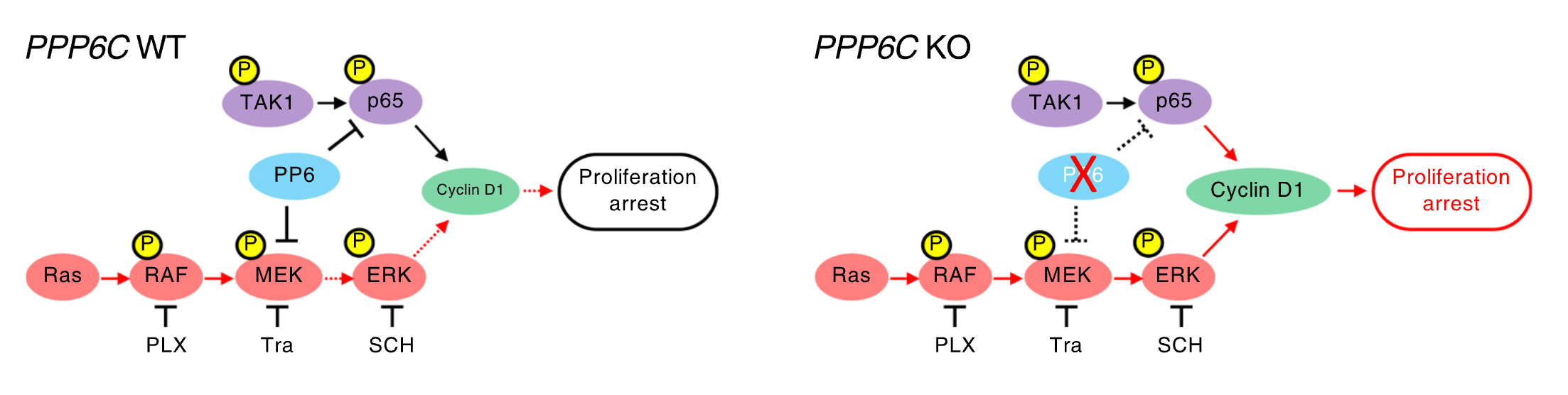
Protein phosphatase 6 activates NF-κB to confer sensitivity to MAPK pathway inhibitors in KRAS- and BRAF-mutant cancer cells.
Zhang H, Read A, Cataisson C, Yang HH, Lee W-C, Turk BE, Yuspa SH, Luo J.
Science Signaling. 2024 May;17:eadd5073. doi: 10.1126/scisignal.add5073
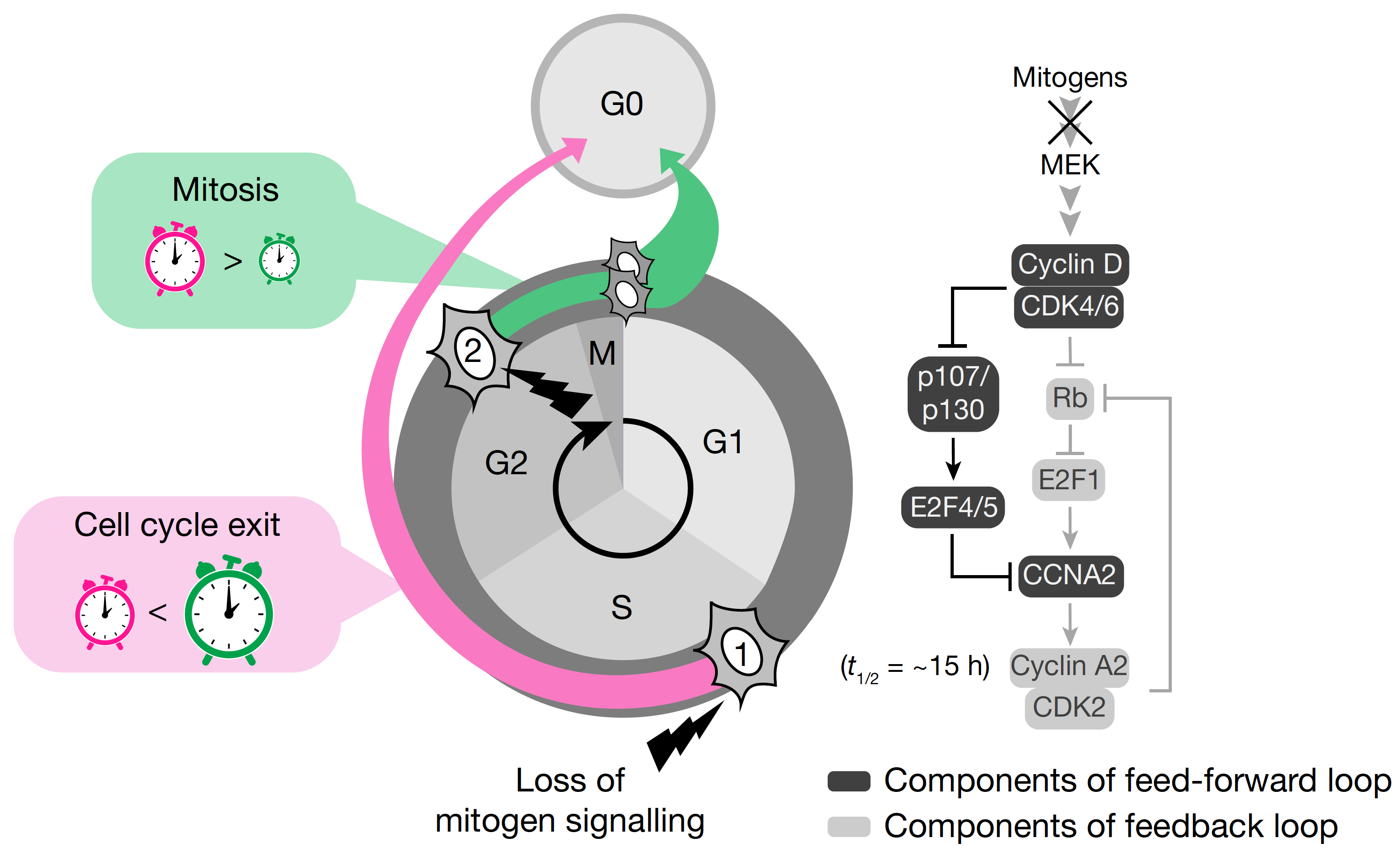
Loss of CDK4/6 activity in S/G2 phase leads to cell cycle reversal.
Cornwell JA, Crncec A, Afifi MM, Tang K, Amin R, Cappell SD.
Nature. 2023 Jul;619(7969):363-370. doi: 10.1038/s41586-023-06274-3