Roberto Weigert, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37, Room 2050-B
- Bethesda, MD 20892
- 240-760-6859
- weigertr@mail.nih.gov
RESEARCH SUMMARY
Dr. Weigert’s research focuses on the basic mechanisms that regulate trafficking events in mammalian tissues, with a particular emphasis on membrane remodeling. He has pioneered subcellular intravital microscopy to image these processes in live animals under physiological conditions, and to investigate their role during tumor progression, invasion, and metastasis. His work has highlighted the principles that control the temporal and spatial coordination in vivo among cell signaling, actin cytoskeleton, and cellular bioenergetics, and their relationship to modifications of the composition and biophysical properties of cellular membranes.
Areas of Expertise

Roberto Weigert, Ph.D.
Research
Neutrophils in action during inflammation in vivo
Membrane trafficking plays a key role in many basic cellular processes and unraveling its molecular machinery is a fundamental step in understanding physiological and pathological events in different tissues. Most of the studies in this field have been carried out in cell and organ cultures. These model systems, that can be easily imaged and manipulated, have provided invaluable information on the mechanisms regulating subcellular events. However, they have major limitations since they do not always recapitulate the complex characteristics of the living tissues in the native environment. To overcome this issue, we have developed subcellular intravital microscopy (IVM), a combination of light microscopy techniques, such as two-photon and confocal microscopy, which enables the direct observation of the intracellular process in live animals. This has been complemented with the development of a series of pharmacological, molecular, and genetic tools that have made possible the investigation of membrane trafficking in vivo at a molecular level.
The two central questions that drive our research are: 1) how membranes continuously change their shape and composition during trafficking events such as endocytosis and exocytosis at the plasma membrane under physiological conditions; 2) how these processes are deregulated during tumor progression, invasion, and metastasis. Specifically, our effort has been directed towards understanding the spatiotemporal coordination of actin cytoskeleton, cell signaling, and cellular metabolism, which is essential in controlling membrane remodeling. To this end, we developed two model systems. The first, based on exocrine glands in live rodents, has been instrumental in unraveling a novel role of an actomyosin complex, which during regulated exocytosis is re-organized at the interface between secretory vesicles and the cell surface in order to drive protein secretion and maintain plasma membrane homeostasis. The second, based on xenografts and carcinogen models for head and neck squamous cell carcinomas, has been instrumental in revealing the role of small GTPases of the Rab family in promoting invasion and metastasis by regulating the dynamics of F-actin at the cell surface. Studies elucidating the signaling networks that elicit the actomyosin-driven membrane remodeling, and the modifications in mitochondrial metabolism, which sustain the bioenergetics of these processes, are in progress.
Publications
- Bibliography Link
- View Dr. Weigert's PubMed Summary.
Dynamic polyhedral actomyosin lattices remodel micron-scale curved membranes during exocytosis in live mice
Intravital microscopy in mammalian multicellular organisms
Concerted actions of distinct nonmuscle myosin II isoforms drive intracellular membrane remodeling in live animals.
Sinusoidal ephrin receptor EPHB4 controls hematopoietic progenitor cell mobilization from bone marrow
In vivo tissue-wide synchronization of mitochondrial metabolic oscillations
Biography

Roberto Weigert, Ph.D.
Dr. Weigert obtained his B.Sc. in chemistry in 1992 from the University of Catania (Italy). He joined the Department of Cell Biology at the Mario Negri Institute (Dr. Alberto Luini) and in 2000 he received his Ph.D. from the Open University of London. In 2001, Dr. Weigert joined the NHLBI at NIH, as a research fellow in the Laboratory of Cell Biology (Dr. Julie Donaldson). In 2006, he joined the NIDCR as chief of the Intracellular Membrane Trafficking Unit, and he was tenured in 2014. In 2015, Dr. Weigert joined the Laboratory of Cellular and Molecular Biology at the NCI. He was appointed Deputy Chief in 2024.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
Covers
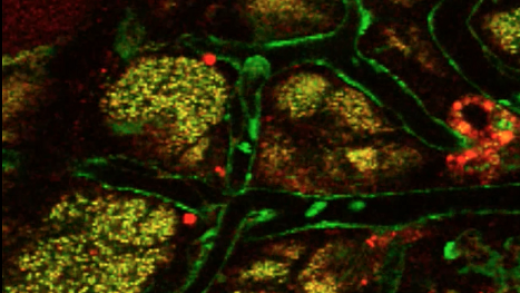
Kinetics of milk lipid droplet transport, growth, and secretion revealed by intravital imaging: lipid droplet release is intermittently stimulated by oxytocin
Description of the cover: The micrograph shows lipid droplets (red) accumulating at the apical surface of secretory cells (green) between oxytocin-induced contractions in a transgenic mouse line that expresses green fluorescent protein in the cytoplasm of most cells.
Abstract: The lipid droplet (LD) fraction of milk has attracted special attention because it supplies preformed lipids for neonatal development, and the assembled LDs are secreted by a unique apocrine mechanism. Because many aspects of this key process remain uncharacterized, we developed a facile method for the intravital imaging of mammary cells in transgenic mice that express fluorescently tagged marker proteins. Using these techniques, we describe the first kinetic analysis of LD growth and secretion at peak lactation in real time. LD transit from basal to apical regions was slow (0–2 μm/min) and frequently intermittent. Droplets grew by the fusion of preexisting droplets, with no restriction on the size of fusogenic partners. Most droplet expansion took several hours and occurred in apical nucleation centers, either close to or in association with the apical surface. Droplets even continued to expand as they were emerging from the cell. Contrary to expectations, LDs attached to the apical plasma membrane but still associated with the cytoplasm were released after oxytocin-mediated contraction of the myoepithelium. Thus milk LD secretion is an intermittently regulated process. This novel procedure will have broad application for investigating trafficking events within the mammary epithelium in real time.
Kinetics of milk lipid droplet transport, growth, and secretion revealed by intravital imaging: lipid droplet release is intermittently stimulated by oxytocin.
Masedunskas A, Chen Y, Stussman R, Weigert R, Mather IH. Mol Biol Cell. 2017 Apr 1;28(7):935-946.