Training at the NIH Clinical Center, the world’s largest biomedical research hospital, provides fellows access to
• an incredibly diverse and unique patient population,
• a mix of standard of care and clinical trial patient care encounters,
• renowned principal investigators,
• first-in-human clinical trials,
• unparalleled research and mentorship opportunities,
• and protected time for a comprehensive, board-focused didactic curriculum.
Jointly supported by the National Cancer Institute’s (NCI) Center for Cancer Research (CCR) and the National Heart Lung and Blood Institute's (NHLBI) Division of Intramural Research, the program is located on the main NIH campus in Bethesda, Maryland, just outside Washington, D.C. Fellows train at the NIH Clinical Center, which places trainees in the midst of limitless opportunities to pursue research in first-in-human trials, drug and cell therapy development, novel therapeutics, machine learning, big data, AI, cancer prevention and screening, healthcare disparities, survivorship, and more.
Research advances and landmarks made at the NIH throughout the years.
Our clinical training is guided by the educational needs of the fellows, not the service needs of the hospital. Fellows have a core set of rotations to cover all key aspects of medical oncology and hematology, with tumor-specific rotations in thoracic, breast-gyn, GI, and GU oncology, as well as rotations in stem cell transplant, transfusion medicine, consultative heme onc, classical hematology, and malignant hematology. Our clinical training also includes offerings that draw on the strengths of the NIH, including rotations in rare tumors, adoptive cell therapy, and early-phase clinical trials.
In addition to the NIH Clinical Center, fellows rotate at major urban hospitals including MedStar Georgetown University Hospital’s Lombardi Comprehensive Cancer Center, George Washington University Hospital, Johns Hopkins Medicine Sidney Kimmel Comprehensive Cancer Center and Sibley Memorial Hospital, University of Maryland Marlene and Stewart Greenebaum Comprehensive Cancer Center, VA Washington, DC Healthcare, Walter Reed National Military Medical Center, and MedStar Washington Hospital Center.
The range of opportunities for individualized training includes the option to complete elective rotations elsewhere. Training at NIH also provides opportunities to learn about the design and management of clinical research and clinical trials, bench-to-bedside research, and population-based research.
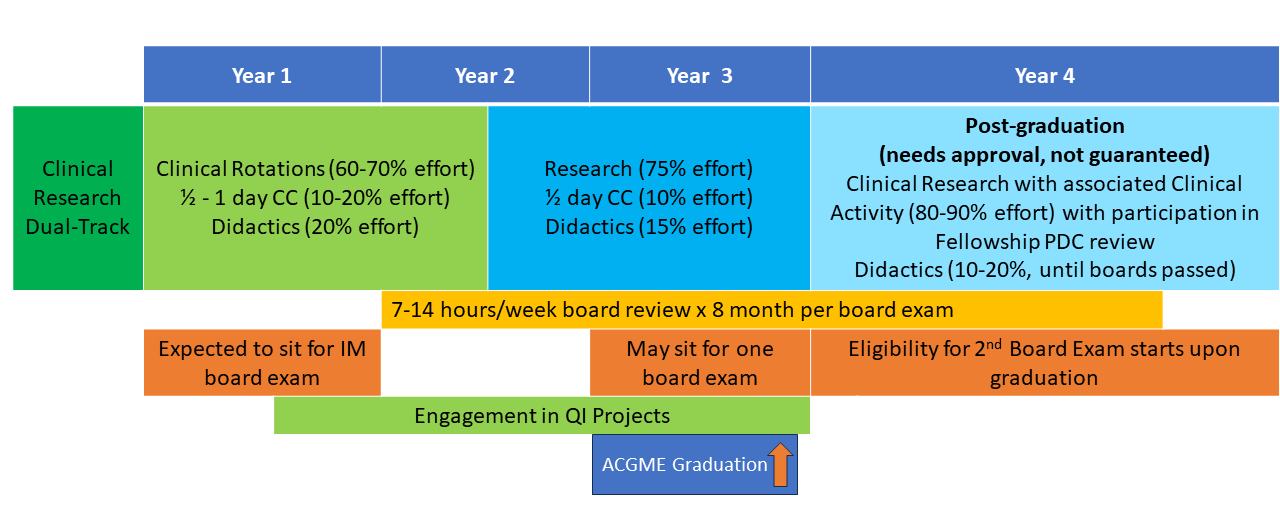
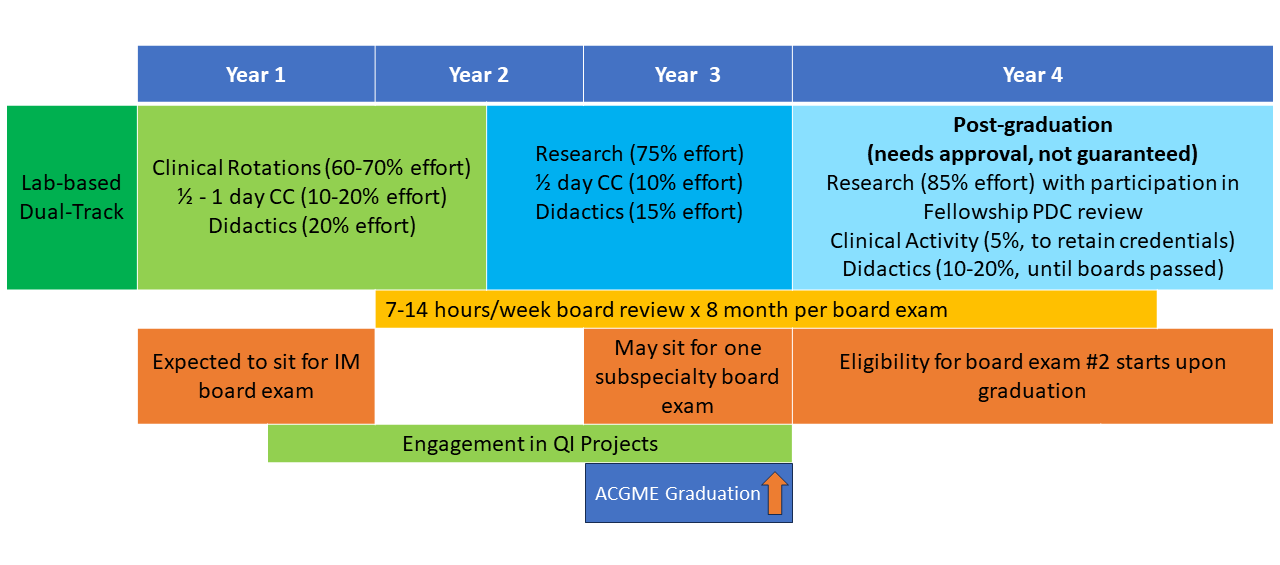
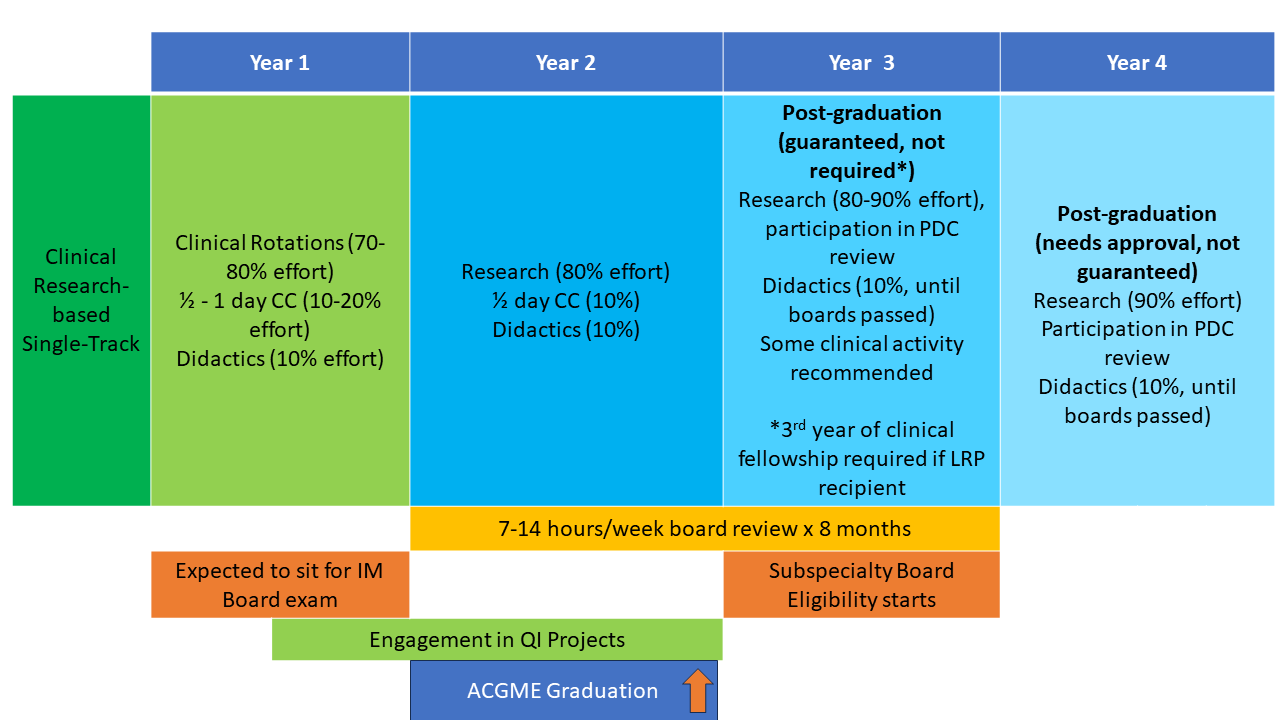
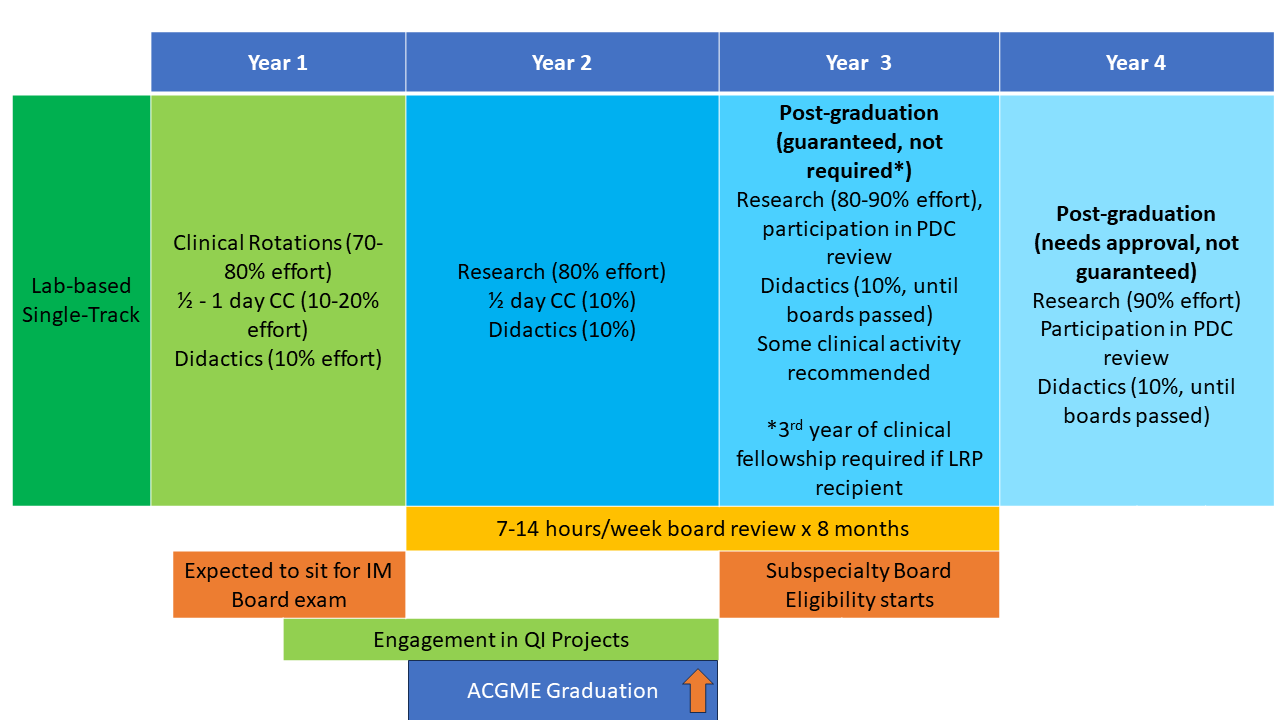
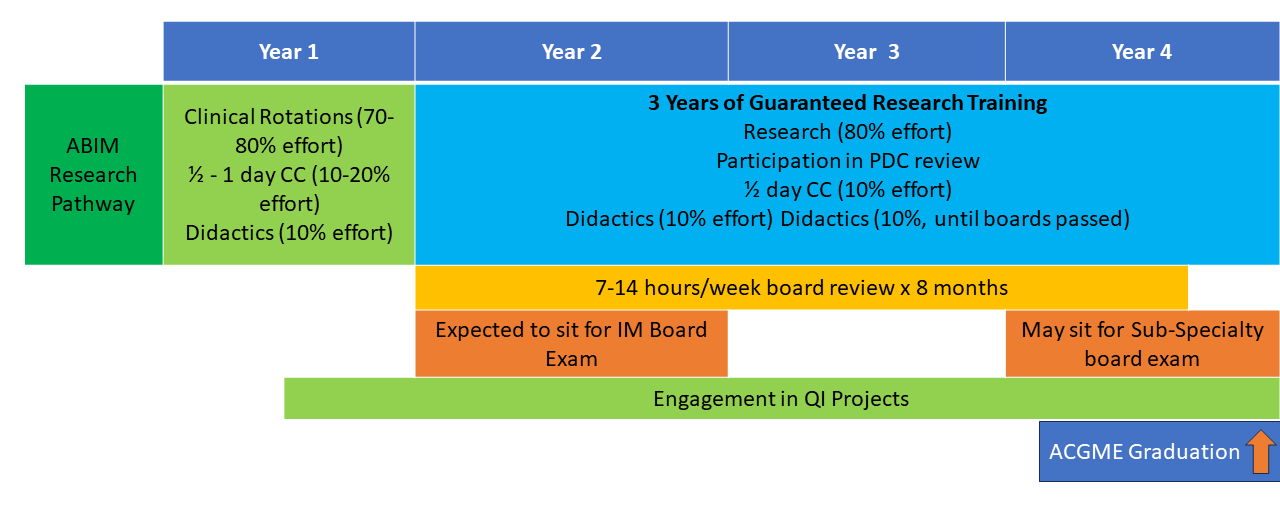
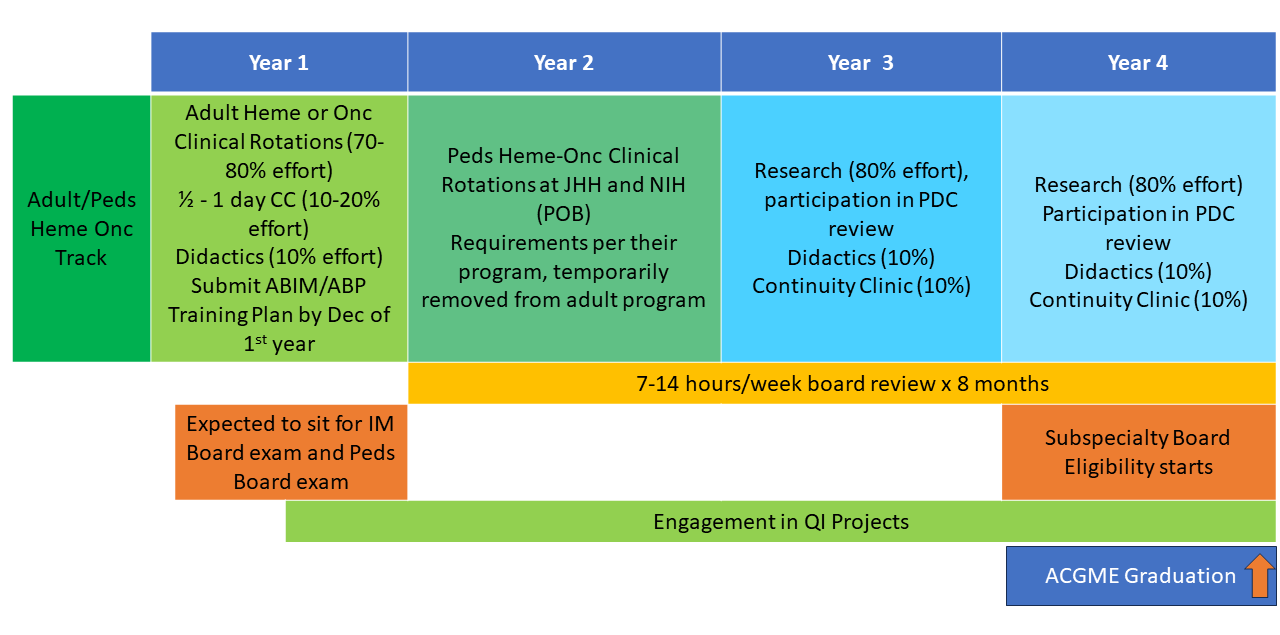
The NIH Hematology Oncology Fellowship program provides a unique opportunity for physicians interested in academic and research careers to develop and integrate their interests in clinical, basic, and population-based research. Fellows may choose to pursue a three-year program (1.5 years of clinical rotations, 1.5 years of research) leading to board certification in both hematology and oncology (dual-track training) or a two-year program (one year of clinical rotations, one year of research) leading to board certification in either hematology or oncology alone (single-track training). All fellows have one half-day of continuity clinic per week throughout their clinical and research time. Scroll down for a schematic showing more about these options.
First and Second Years: The first 12-18 months of clinical training includes rotations on the inpatient wards, as well as outpatient clinics at the NIH Clinical Center in hematopoietic stem cell transplantation, lymphoma, leukemia, solid-tumor oncology, bone marrow failure, sickle cell hemoglobinopathies, and hematology/oncology consults. Additional structured clinical rotations are performed in the clinics listed in the above “Clinical Excellence” section.
Second and Third Years: In the final 12-18 months of fellowship during their protected research time, fellows acquire the skills necessary to become independent biomedical investigators. They may choose to work with one of the more than 100 laboratories and clinical research groups at the NIH. The choice of laboratory or clinical research group is made by mutual agreement of the fellow, the laboratory or clinical mentor, and the fellowship leadership. During this period, the half-day/week clinic continuity obligation continues, along with didactic activities and further development of clinical independence.
Fellows may elect to work with any investigator on the NIH campus, not only those within the NCI or the NHLBI. Some fellows choose to continue their research time beyond the three-year program to enhance their competitiveness for intramural tenure-track positions or extramural positions and grant funding.
NIH Hematology Oncology Fellows can participate in additional training opportunities within other parts of the NIH as well as with outside institutions.
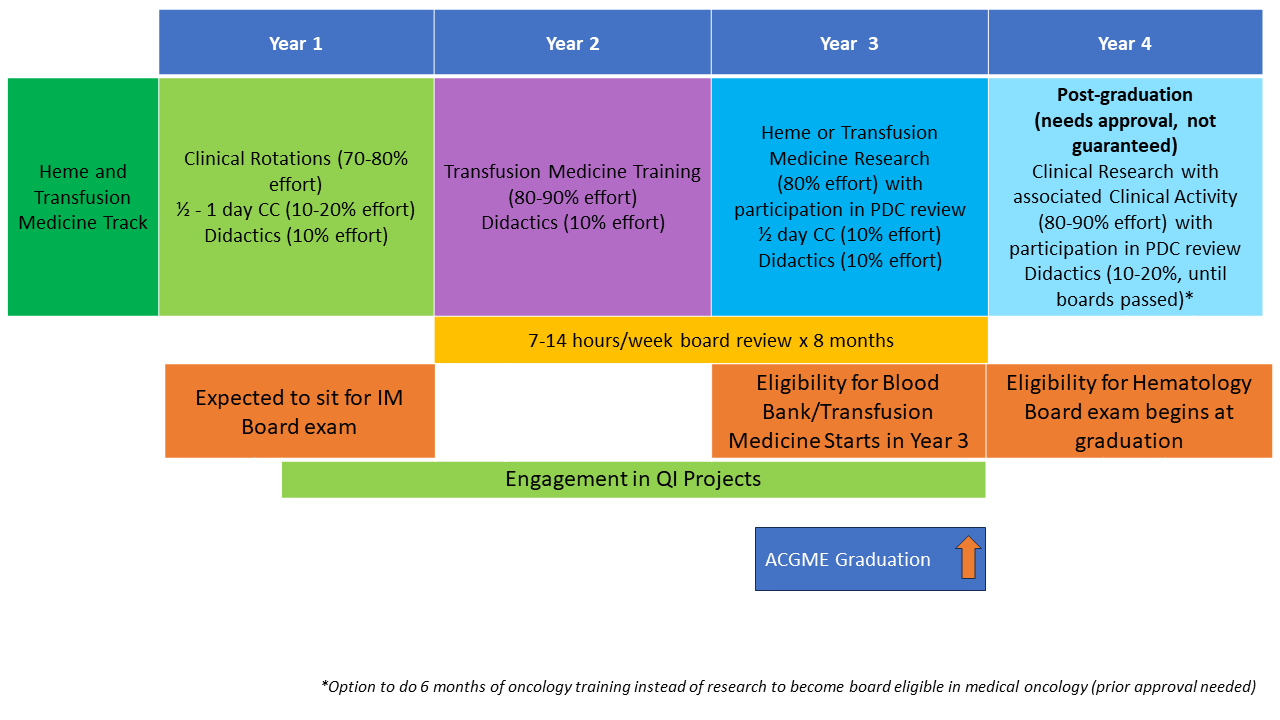
Combined Hematology and Transfusion Medicine/Blood Banking Fellowship: This new training option, named in honor of Dr. Elaine M. Sloand, allows fellows to complete training resulting in degrees and certification in both blood banking/transfusion medicine and hematology (+/- medical oncology). The program is three years for single-track hematology fellows and four years for those interested in dual-track hematology oncology training. Applicants should make their interest in this track known at the time of fellowship application through ERAS/NRMP. Learn more
Interagency Oncology Task Force (IOTF) Fellowship: Offered as a partnership of the NIH, the U.S. Food and Drug Administration (FDA), and the U.S. Department Health and Human Services (HHS), the IOTF program trains scientists in research and research-related regulatory review, policies, and regulations to develop a skillset that bridges the two disparate processes. The program trains physicians in aspects of clinical trials methodology and analysis, epidemiology, clinical aspects of medical product development, and regulation to facilitate the movement of drugs, biologics, and devices from basic bench science to commercialization. Any NIH hematology oncology fellow may apply for this program. Learn more
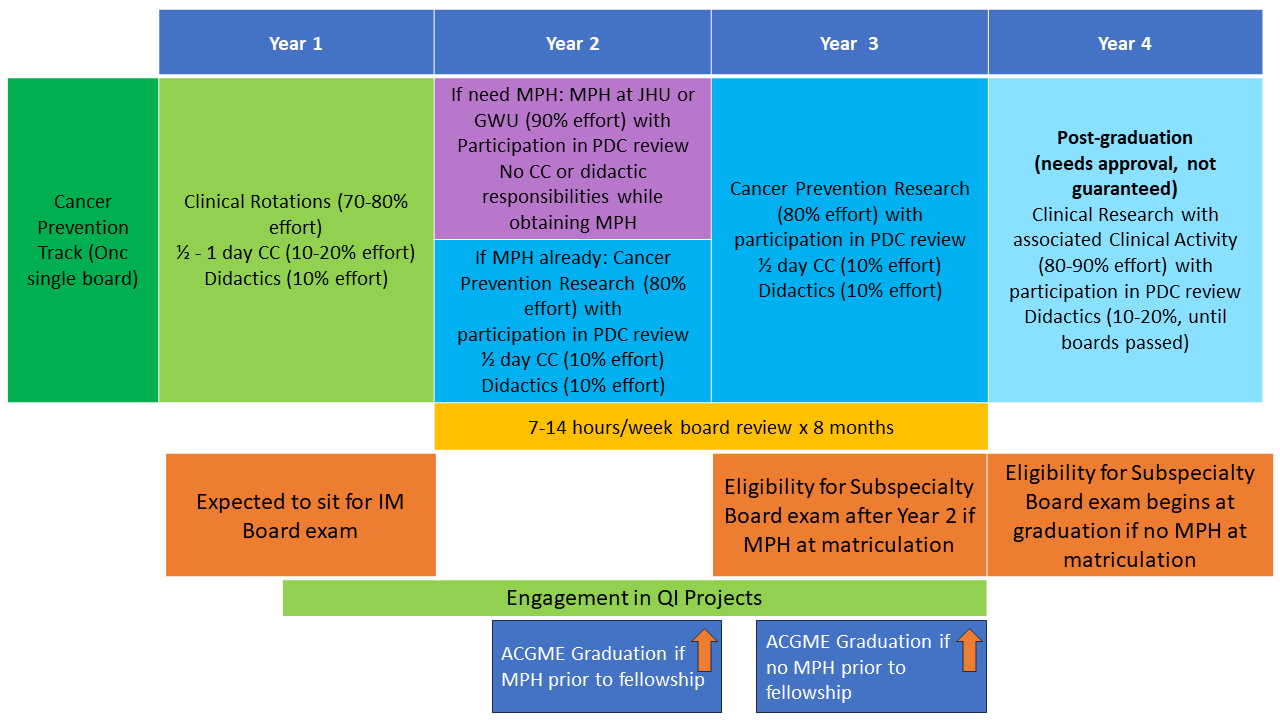
Joint Training in Cancer Prevention & Control: A collaborative effort between the NIH Hematology Oncology Fellowship and the NCI Cancer Prevention Fellowship Program (CPFP) allows single-track medical oncology fellows to complete both clinical training in oncology and research training in cancer prevention and control. The Joint Training in Cancer Prevention and Control track provides clinical training with the NIH Hematology Oncology Fellowship Program, the opportunity to earn a fully funded MPH or equivalent degree sponsored by the CPFP, mentored research opportunities in cancer prevention and control across the NCI, and access to a structured professional development curriculum. Those interested should apply during the initial fellowship application process in ERAS and match through the NRMP. For more information about this track, the training timeline, and the CPFP, please visit the CPFP website.
Joint Training in Clinical Trials Research: During their second or subsequent years, hematology and/or medical oncology fellows may also apply to participate in a formal training program in clinical trials research, offered collaboratively with Duke University and leading to a Master of Health Sciences in Clinical Research (MHSc) degree. Any NIH Hematology Oncology fellow may decide to apply to participate during fellowship. Learn more
The fellowship has protected time for didactics on Fridays each week, and fellows from all years attend. Led by alumni of our program and featuring faculty from throughout NIH as well as outside experts, our didactics feature innovative educational techniques and comprehensively cover both standard of care approaches and breakthrough advances in hematology and oncology. All sessions are recorded for later study and review. Our interim director, Christopher Pleyer, M.D., leads regular "pulse-taking" sessions soliciting feedback from the fellows with a focus on well-being and program improvement. Stacey Doran, M.D., our associate program director for medical oncology, co-leads this session.
The types of learning activities the fellowship provides include:
- Hematology and Oncology core lectures
- Bootcamp for new fellows
- Tumor Boards and discussions
- Walk Rounds
- Case-based lectures
- White Board Talks in clinic
- Fellow-led breakout sessions
- Board Review Questions/Trivia/Jeopardy, including fellow-led reviews
- Flipped Classroom
- Pharmacology lectures by NIH and outside experts
- Journal Club led by a biostatistician
- Pulse-taking sessions led by fellowship leadership
- Best of ASCO and ASH lectures
- Job talks by senior fellows
- Workshops on biostatistics and palliative care
- Morbidity and Mortality
- Chalk Talks led by fellows