NCI Immunotherapy Fellowship
NCI Immunotherapy Fellowship
About
Overview
This training program is designed for physicians who have completed a medical oncology fellowship program in the United States and who seek specialized training in immunotherapy with clinical trials and clinical trial development.
There are multiple opportunities for training in clinical immunotherapy at the Center for Cancer Research (CCR), National Cancer Institute (NCI), with active clinical programs in therapeutic cancer vaccines, immune checkpoint modulation, adoptive cellular therapies (both TCR transgenic and CAR-T therapy) and antibody-based immunotherapies. This training program, at the CCR in Bethesda, Maryland, allows the fellow to have exposure to multiple clinical immunotherapeutic approaches and also to key opinion leaders in the field of clinical immunotherapy. This 1-year fellowship provides opportunities to understand how to design, write and run clinical trials, how to treat patients, how manage toxicities, as well as opportunities to work with multiple experimental agents.
Funding and Co-Sponsorship
This fellowship is co-sponsored by the National Cancer Institute and the Society for Immunotherapy of Cancer (SITC) and made possible, in part, by an educational grant from EMD-Serono.
How to Apply
To apply, please send your curriculum vitae, statement of purpose, three personal references, and your documentation of board eligibility status or certification status to education@sitcancer.org
Application Period Opens: July 8, 2024 - August 12, 2024
Review Period: September 2024
Notification Date: Oct. 7, 2024 - Oct. 11, 2023
Program Director
Danielle Pastor, D.O., Ph.D.
Center for Immuno-Oncology
240.858.3737
Background
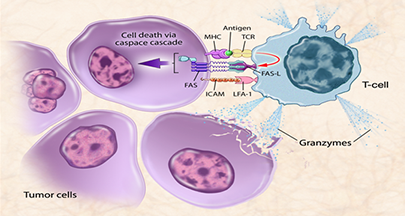
Immunotherapy for cancer has fundamentally changed the way we are treating patients with metastatic disease. The exploding interest in immunotherapy has been propelled by the rapid and durable responses seen in treated patients, often with little to no side effects. Billions of dollars are now being poured into immunotherapy research with multiple new approvals since 2010 and even more expected in the near future. However, the basics of immunotherapy, taught in medical schools when our current generation of medical oncologists trained, are inadequate for the high-tech immunotherapies being tested with ever-increasing intensity. In addition, the side effect profile with immunotherapies is related to the mechanism of action and is unique among anti-cancer agents. The mechanism of action is more complex as often agents don’t directly target the cancer but rather target the immune system which, in turn, targets the cancer. Furthermore, the kinetics of a clinical response following immunotherapy may be different from conventional therapy which is important for practitioners and patients alike to know to help manage expectations. Finally, the mechanisms of resistance to immunotherapy are also unique and a better understanding of these mechanisms will guide combination therapy.
Benefits
- Work with internationally recognized experts in immunotherapy
- Learn management of immune-related adverse events
- Develop experience in writing and running immunotherapy clinical trials
- Develop experience with a wide array of immunotherapy approaches: immunue checkpoint inhibitors, adoptive cellular therapy (CAR-T or TCR transgenic approaches), therapeutic vaccines, immunocytokines, and combination immunotherapy approaches
- Possibilities for attending and presenting at national/international meetings
- Attend and participate in regularly scheduled NIH immunotherapy conferences
- Make a difference in the lives of patients with cancer at the largest hospital in the world devoted solely to research
- Salary is based on applicable laws, regulations, and policies