Laboratory of Molecular Radiotherapy
Freddy E. Escorcia, M.D., Ph.D.
Laboratory of Molecular Radiotherapy
Research
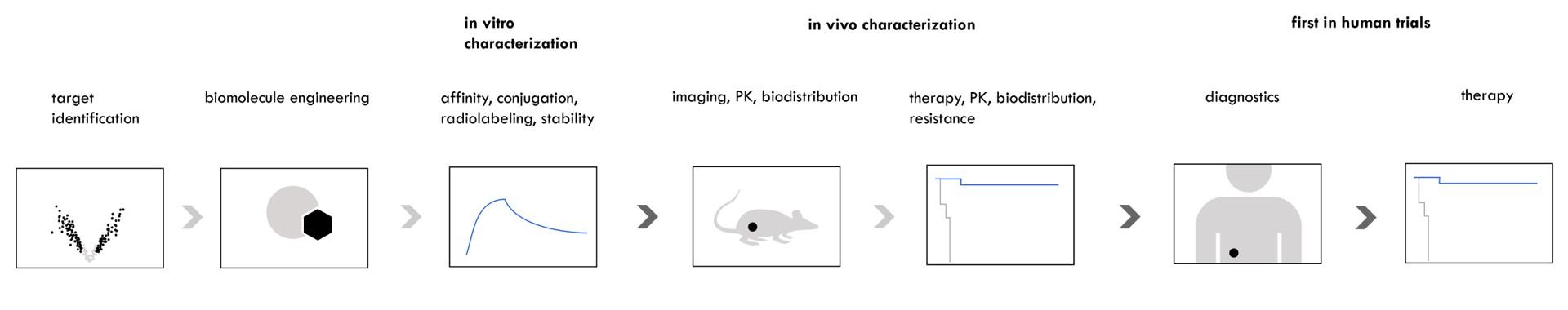
We leverage modern technology to achieve the following:
- Identify promising tumor- or tumor microenvironment-selective targets
- Discover low molecular weight biomolecules specific to these targets
- Design, engineer and test radioconjugates in vitro and in vivo
- Translate lead radioconjugates to first in human clinical trials
Current efforts are focused on engineering radioconjugates to yield molecular PET or SPECT imaging agents for hepatocellular carcinoma (HCC), which is critical in early diagnosis and surveillance of this disease and suboptimal with existing conventional methods, especially following local treatments. Importantly, our efforts toward the development of novel imaging agents for HCC yield insights which we can use to inform development of therapeutic agents.
Specifically, we have been exploiting the HCC-selective marker Glypican-3 (GPC3), which is expressed in 75-90% of HCC, and is being investigated in clinical trials for targeted therapy, including an NCI clinical trial (NCT05003895) evaluating chimeric antigen receptor T (CART) cells specific to GPC3 in patients with metastatic HCC. In our lab, we have identified a range of novel biomolecules with specificity to GPC3 that we are characterizing and testing for applications as imaging and therapeutic radiopharmaceuticals in preclinical studies. Our goal is to eventually translate the most promising agent(s) to first in human studies. Until that point, we are also exploring repurposing of existing imaging and therapeutic radiopharmaceuticals being used in other cancers to help in patients with HCC. An example of this approach is using the prostate specific membrane antigen (PSMA) imaging agent 18F-DCFPyL that is approved for men with prostate cancer. Because PSMA is also expressed in the neovasculature of HCC, we are assessing the positive predictive value of this tracer for HCC in a clinical trial (NCT05009979). In additional the trial is evaluating whether the tracer can help distinguish viable from non-viable tumor.
While our focus is on radiopharmaceuticals, what we learn can be translated to similar technologies including antibody- or peptide-drug conjugates, and have applications beyond HCC as well.
Additionally, because we know that radiopharmaceutical therapy is unlikely to result in cures, future efforts in identifying relevant targetable cellular resistance pathways will help guide rational treatment combinations to improve the outcomes for patients.