
Craig J. Thomas, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building B, Room 3005
- Rockville, MD 20850-3370
- 301-217-4079
- craigt@mail.nih.gov
RESEARCH SUMMARY
Craig Thomas uses high-throughput technologies to make novel discoveries at the interface of chemistry and biology. Dr. Thomas serves as leader of chemistry technologies at the NIH National Center for Advancing Translational Sciences (NCATS). His research interests include the production of novel screening libraries, the development of biologically active small molecules, the association of phenotype to pharmacology, the phenotypic screening of mechanistically annotated small molecule libraries, the development of novel drug combinations, and the progression of agents into advanced pre-clinical and clinical studies.
Areas of Expertise
Research
The Thomas lab works at the interface of chemistry and biology mixing pharmacology, chemical biology, medicinal chemistry, systems biology and drug development to explore new translational opportunities across a range of disease states. Scientists in our laboratory use state-of-the-art technologies to conduct large chemogenomic screens of approved and investigational drugs in single agent and combination format as a means to define drug response signatures in multiple disease models. The outcomes of these studies span from basic to translation research. In the domain of basic research, the outcomes of these studies reveal new insights in pharmacology-informed systems biology. On the translational front, the outcomes of these studies can define new therapeutic opportunities for clinical development. Our medicinal chemistry and drug discovery efforts employ a mix of structure-based and high-throughput methods to identify chemical leads which are then optimized into biologically active probes of key cellular targets. These new, biologically active probes provide important tools that interrogate the functional consequences (phenotypes) associated with inhibiting/activating the targets of interest in key disease models. These tools are offered freely. Occasionally, agents that result from these efforts qualify as investigational drugs and enter human evaluation.
Publications
- Bibliography Link
- View Dr. Thomas's Complete Bibliography at NCBI.
Biography

Craig J. Thomas, Ph.D.
Craig Thomas received his B.S. from the University of Indianapolis in 1995 and his Ph.D. from Syracuse University in 2000. He then completed postdoctoral work in the laboratories of Sidney Hecht, where he earned a fellowship through the American Cancer Society. In 2003, Thomas joined NIH as director of the chemical biology core at the National Institute of Diabetes and Digestive and Kidney Diseases. In 2007, he moved to the NIH Chemical Genomics Center, which was supported by the National Human Genome Research Institute. The center now is supported by the NIH National Center for Advancing Translational Sciences (NCATS) and is called the NCATS Chemical Genomics Center. He is an adjunct member of the National Cancer Institute’s Chemical Biology Laboratory and an adjunct associate professor at the Georgetown University School of Medicine and the Temple University School of Medicine. Thomas is an editor of Current Protocols in Chemical Biology and a member of the SciFinder (Chemical Abstracts Service) advisory board. He has published more than 100 peer-reviewed manuscripts, is an inventor on more than 15 patents and has given numerous invited lectures.
Covers
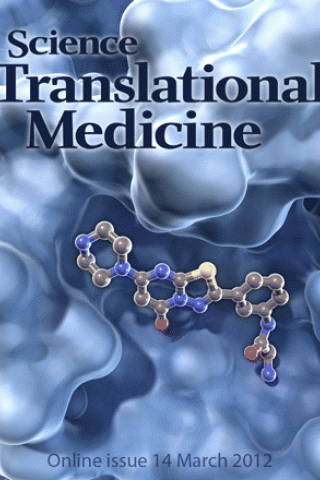
Structure-Guided Design of a High-Affinity Platelet Integrin αIIbβ3 Receptor Antagonist That Disrupts Mg2+ Binding to the MIDAS
A Better Fit. An improved anticoagulant drug called RUC-2 (ball and stick structure) fits snugly into its binding pocket on integrin (blue), a protein found on the surface of platelets. RUC-2 binds both subunits of integrin, inhibiting the excessive blood coagulation that can lead to strokes and heart attacks. Unlike similar drugs that alter integrin's structure when they bind and trigger unwanted immune responses, RUC-2 does not disturb the configuration of its larger partner.
See: Structure-Guided Design of a High-Affinity Platelet Integrin αIIbβ3 Receptor Antagonist That Disrupts Mg2+ Binding to the MIDAS by Jieqing Zhu, Won-Seok Choi, Joshua G. McCoy, Ana Negri, Jianghai Zhu, Sarasija Naini, Jihong Li, Min Shen, Wenwei Huang, Daniel Bougie, Mark Rasmussen, Richard Aster, Craig J. Thomas, Marta Filizola, Timothy A. Springer and Barry S. Coller in the Science Translational Medicine, 2012, 4(125), 125ra32. [CREDIT: C. BICKEL/SCIENCE TRANSLATIONAL MEDICINE].