CAR T-Cell Therapy
Photo courtesy of NCI Visuals Online
Patients with multiple myeloma that has not responded to standard treatment may be eligible to participate in a new clinical trial at the NIH Clinical Center.
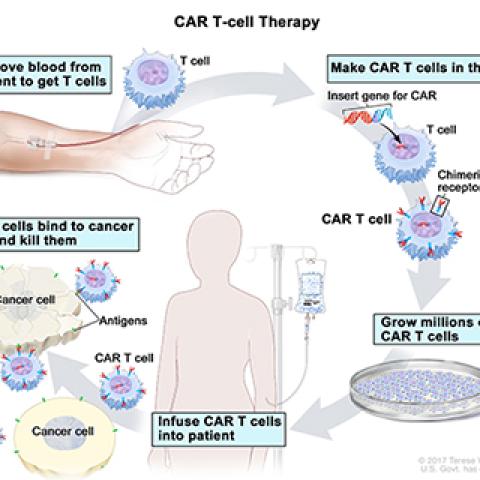
James N. Kochenderfer, M.D., Investigator in the Surgery Branch, is leading a study of a new way to treat multiple myeloma (MM) that uses a patient’s own T cells to target MM cells. MM is a rare blood cancer that occurs in blood, tissues, bone and bone marrow. With MM, a group of plasma cells become cancerous and multiply, crowding out healthy blood cells. In this trial, investigators are studying a new way of treating MM using patients’ own T cells. Patients undergo a process called leukapheresis where blood is removed from the patient and T cells are extracted. The T cells are sent to a lab where they are genetically altered to become chimeric antigen receptor (CAR) T cells. These CAR T cells have receptors that recognize and target a specific protein (SLAMF7) that appears on the surface of MM cells. The anti-SLAMF7 CAR T cells are also fitted with a genetic stop switch. In case of severe side effects from the treatment, a second drug will be administered that activates a switch that shuts down CAR T cells and eliminates them from the patient’s body. The goal of this study is to see if anti-SLAMF7 CAR T cells with a stop switch are safe to give to patients with MM.
Clinicaltrials.gov identifier: NCT03958656
NCI Protocol ID: NCI-19-C-0102
Official Title: A Phase I Clinical Trial of T Cells Expressing an Anti-SLAMF7 CAR for Treating Multiple Myeloma
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.