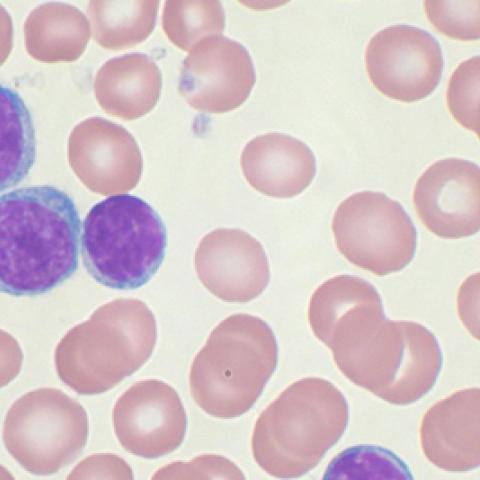
High-power magnification (10006X) of a Wright's stained peripheral blood smear showing chronic lymphocytic leukemia (CLL). The lymphocytes with the darkly staining nuclei and scant cytoplasm are the CLL cells.
Photo credit: Wikimedia Commons
Patients with chronic lymphocytic leukemia (CLL) that has not responded to standard therapy may be eligible to participate in a new clinical trial at the NIH Clinical Center.
CLL a type of cancer that develops in blood-forming cells (B cells or lymphocytes) in the bone marrow. These leukemia cells build up in the bone marrow and crowd out normal cells. Eventually, the malignant B cells spill into the bloodstream and spread to other organs. Milos Miljkovic, M.D., of the Lymphoid Malignancies Branch, is leading a trial that uses a combination of IL-15 and obinutuzumab to treat CLL that has not responded to standard treatment.
Cancer patients can have weak immune systems. IL-15 is a man-made version of a natural protein that can boost the immune system’s ability to fight cancer. Obinutuzumab is a monoclonal antibody (produced by identical immune cells cloned from a parent cell) that attaches to a specific antigen on leukemia cells. The body’s natural immune cells are then recruited to attack and kill the marked leukemia cells. The goal of the study is to determine the safe dose of this drug combination, identify any side effects and see if this treatment has a beneficial effect on CLL.
Clinicaltrials.gov identifier: NCT03759184
NCI Protocol ID: NCI-19-C-0024
Official Title: Pilot Trial of Human IL-15 (rhIL-15) and Obinutuzumab for Refractory and Relapsed Chronic Lymphocytic Leukemia (CLL)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.