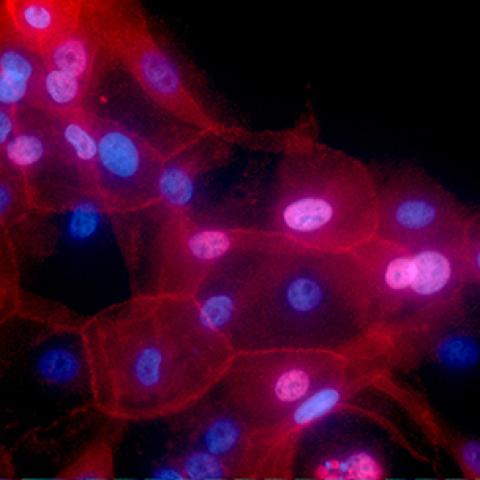
The image shows cell culture of human breast cancer conditionally reprogrammed cells. Fluorescence red color represents MHC-I, and nuclei are shown in blue.
Image source: NCI Visuals Online
Adults with locally advanced or metastatic solid tumors that cannot be treated with surgery may be eligible to participate in a clinical trial at the NIH Clinical Center.
Researchers have explored several different combinations of immunotherapies to see if they improve responses in people with cancer. James L. Gulley, M.D., Ph.D., Chief of the Genitourinary Malignancies Branch, is leading such a study in people with solid tumors that have spread to other parts of the body. Participants will begin by taking SX-682, which investigators hope will inhibit tumor cells’ ability to grow and spread to other sites in the body. If participants do well on SX-682 alone, they will then get bintrafusp alfa and BN-CV301. Bintrafusp alfa simultaneously blocks two signals that cancer cells use to avoid being attacked and destroyed by immune cells. BN-CV301 is a cancer vaccine that is made from viruses whose genes have been modified to make them safe and able to carry cancer drugs to tumor cells. After investigators determine that the combination of these immunotherapies is safe, the study will focus on patients with certain types of breast cancer and head and neck cancer. Patients will then receive all three treatments at the same time.
Clinicaltrials.gov identifier: NCT04574583
NCI Protocol ID: NCI-20-C-0155
Official Title: Phase I/II Trial Investigating the Safety, Tolerability, Pharmacokinetics, Immune and Clinical Activity of SX-682 in Combination with BinTrafusp Alfa (M7824 or TGF-beta "Trap"/PD-L1) With CV301 TRICOM in Advanced Solid Tumors (STAT)
The Center for Cancer Research (CCR) is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.