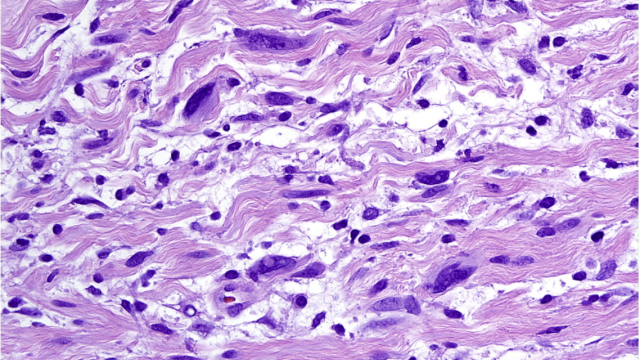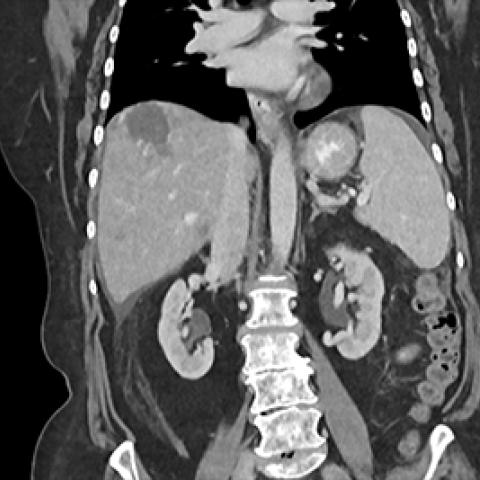
Patient with urachal adenocarcinoma (a rare form of bladder cancer) with liver metastases enrolled in the ICONIC trial.
Image courtesy of Andrea Apolo
Patients with rare, metastatic genitourinary malignancies may be eligible to participate in a new clinical trial at the NIH Clinical Center.
Some cancers of the genitourinary tract are so rare that it is difficult to study them systematically in large clinical trials. To address this issue, Andrea B. Apolo, M.D., of the Genitourinary Malignancies Branch, is leading a study to recruit patients with each of the nearly 40 rare genitourinary malignancies and treat them with a combination of chemotherapy and immunotherapy drugs. This study will test the effectiveness of two immunotherapy drugs (nivolumab and ipilimumab) with one anticancer targeted drug (cabozantinib) for rare genitourinary tumors. Cabozantinib, a targeted therapy, blocks certain proteins that may help keep cancer cells from growing. Nivolumab and ipilimumab are immunotherapy drugs that are meant to help the body’s own immune system attack cancer cells. The goal of this study is to see if combining these three drugs leads to improved antitumor responses.
Clinicaltrials.gov identifier: NCT03866382
NCI Protocol ID: NCI-19-C-0137
Official Title: A Phase II Study of Ipilimumab, Cabozantinib, and Nivolumab in Rare Genitourinary Cancers (ICONIC)
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.