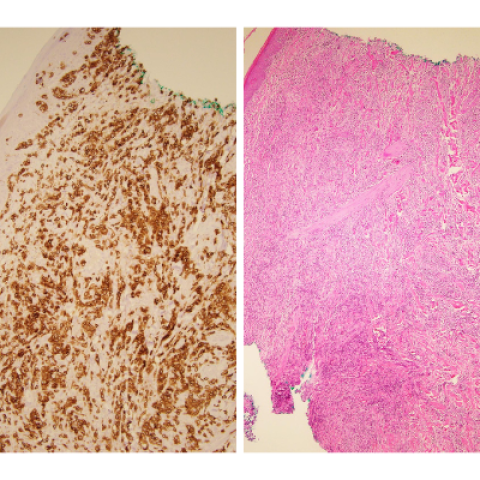
A CD3 immunostain (left) and an H&E of a skin biopsy (right) from a patient with cutaneous T-cell lymphoma.
Image credits: Stefania Pittaluga, M.D., Ph.D., Laboratory of Pathology
Adults with a T-cell malignancy that has not responded to, or has returned after, at least one line of therapy may be eligible to participate in a clinical trial at the NIH Clinical Center.
T cells play a central role in the body’s immune response and cancer surveillance but can themselves become cancerous. T-cell malignancies (TCMs) are a rare and varied group of leukemias and lymphomas originating from T-cells. Patients with TCMs are usually treated with a combination of chemotherapy drugs that help only about a third of cases. If the disease comes back after this treatment regimen, the prognosis is very poor. Milos Miljkovic, M.D., M.Sc., Assistant Research Physician in the Lymphoid Malignancies Branch, is leading a study of a treatment strategy for TCMs. This regimen is a 4-drug combination of romidepsin, oral 5-azacitidine, dexamethasone, and lenalidomide. Romidepsin interferes with the genetic makeup of cancer cells, slowing the growth of cancer cells, leading to cell death. Azacitidine gets incorporated into cancer cells’ DNA. When this happens, the cells cannot function properly and also results in cell death. Dexamethasone is a corticosteroid similar to a natural hormone produced by the adrenal glands and relieves inflammation. Lenalidomide inhibits the growth of blood vessels in tumors, ultimately leading to cell death. Researchers are seeking to determine the safety, side effects, and best dose of this 4-drug combination for people with relapsed/refractory TCM.
Clinicaltrials.gov identifier: NCT04447027
NCI Protocol ID: NCI-20-C-0127
Official Title: A Phase 1 Study of Romidepsin, CC-486 (5-azacitidine), Dexamethasone, and Lenalidomide (RAdR) for Relapsed/Refractory T-cell Malignancies
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.