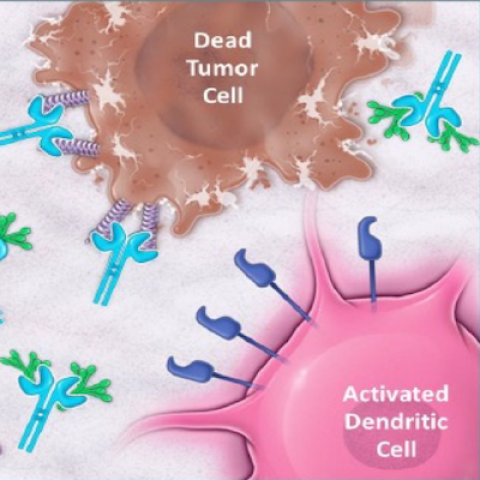
NC410 binds to tumor-associated ligands (collagen and C1q) to block LAIR-1 inhibition and promote adaptive (T cells) and innate (dendritic cells) immune responses, as well as to activate macrophages, ultimately resulting in tumor cell killing.
Image courtesy of NextCure, Inc.
A new multicenter study is enrolling adults with advanced or metastatic solid (non-blood) tumors that have not responded to standard treatments. Marijo Bilusic, M.D., Ph.D., Associate Research Physician in the Genitourinary Malignancies Branch, is leading NCI’s participation in the study. Tumor cells have many different ways of inhibiting the function of T cells, the main weapon of the immune system, or evading T cells altogether. One of these ploys involves a protein called LAIR-1 that inhibits immune function and prevents an optimal immune response. In pre-clinical studies, an experimental drug, NC410, blocked the ability of LAIR-1 to suppress the immune system and significantly increased normal immune function and antitumor T-cell activity. In this phase 1 study, participants will receive NC410 and researchers will closely monitor any serious side effects. They will also determine the best dose of NC410 that will achieve an antitumor response. Researchers believe that NC410 may be able to help patients with solid tumors who are not being helped by currently available therapies.
Clinicaltrials.gov identifier: NCT04408599
NCI Protocol ID: NCI-20-C-0098
Official Title: A Phase 1/2, Open-Label, Dose-Escalation, Safety and Tolerability Study of NC410 in Subjects With Advanced or Metastatic Solid Tumors
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.