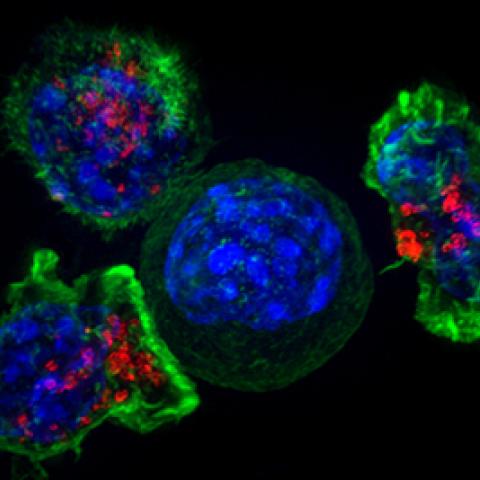
Superresolution image of a group of killer T cells (green and red) surrounding a cancer cell (blue, center).
Photo courtesy of NCI Visuals Online
Patients with advanced mesothelin-expressing solid tumors may be eligible to participate in a new clinical trial at the NIH Clinical Center.
Mesothelin is a protein found on the surface of normal, healthy cells that may help the cells stick together and send signals. Some cancer cells express a higher-than-normal amount of mesothelin, which makes them more likely to multiply and spread to other parts of the body. Raffit Hassan, M.D., of the Thoracic and GI Malignancies Branch, is leading a trial that tests T-cell immunotherapy for patients with cancer of the lung and its lining, ovarian cancer and bile duct cancer that express high levels of mesothelin. This therapy harnesses a patient's T cells, altered to recognize mesothelin, with the aim of attacking cancer cells.
Clinicaltrials.gov identifier: NCT03907852
NCI Protocol ID: NCI-19-C-0117
Official Title: A Phase 1/2 Single Arm Open-Label Clinical Trial of TC-210 T Cells in Patients With Advanced Mesothelin-Expressing Cancer
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.