As emerging imaging platforms change how we see prostate cancer, clinical trials are required to understand how they can be used to optimize patient care.
Image courtesy of Ravi Madan
Men who have been treated for localized prostate cancer but still have signs of the disease in their blood may be eligible to participate in a clinical trial at the NIH Clinical Center.
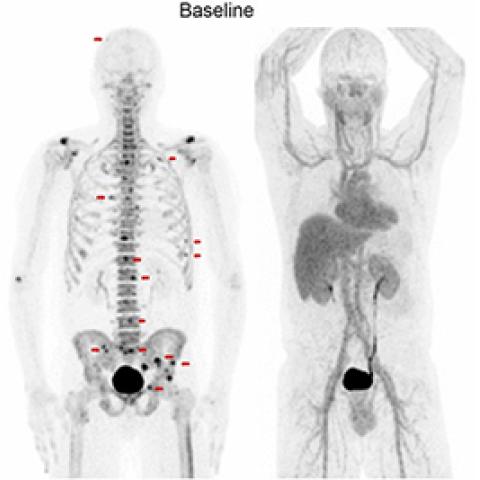
Some men who have been treated for localized prostate cancer (PC) with surgery or radiation still have signs of the disease that are only detected by blood test (a rising prostate specific antigen or PSA). This is called biochemically recurrent prostate cancer (BCRpc). Men with BCRpc may also have micrometastatic disease, which is too small to be seen with conventional imaging. One treatment option for men with BRPC is hormonal therapy, which can have toxic side effects. In this study, Ravi A. Madan, M.D., Senior Clinician in the Genitourinary Malignancies Branch, is exploring an option meant to be less toxic for treating BCRpc, which may impact microscopic bone disease seen only on PET scans. Radium-223 is a mildly radioactive form of the metal radium. Besides killing cancer cells, this form of radiation may positively affect the immune system by boosting its ability to recognize and kill cancer cells with activated T cells. This study will help investigators understand the treatment’s effect on men with micrometastatic PC that is too small to be detected with standard imaging, in addition to learning how radium-223 affects the immune system.
Clinicaltrials.gov identifier: NCT04206319
NCI Protocol ID: NCI-20-C-0010
Official Title: Phase II Trial of Radium-223 in Biochemically Recurrent Prostate Cancer
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.