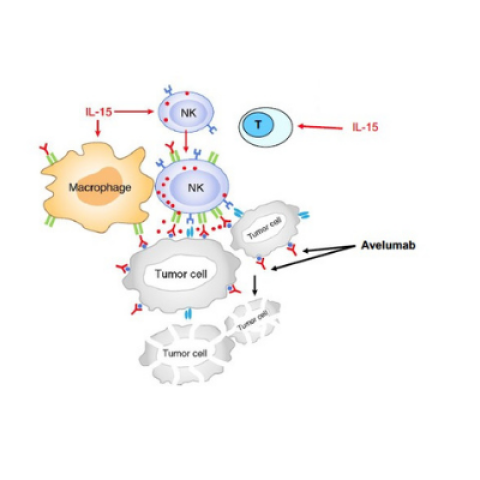
Adapted “Schema of in vivo working model between T-cells, NK cells and macrophages and Avelumab during antitumor immune response.” (Meili Zhang et al. PNAS 2018;115:46:E10915-E10924)
Patients with clear cell renal carcinoma (ccRCC) that has spread or returned after at least two prior therapies may be eligible to participate in a clinical trial at the NIH Clinical Center.
Kevin Conlon, M.D., Associate Research Physician in the Lymphoid Malignancies Branch, is leading a study to see how well patients with relapsed refractory metastatic ccRCC respond to a combination Avelumab and interleukin (IL)-15 treatment. Kidney cancer is the 6th most common cancer diagnosed in the United States with greater than 73,000 new cases seen each year. ccRCC is the most common subtype of kidney cancer and has been given this name because the malignant cells have a distinctive empty, or clear, appearance when viewed under the microscope. When ccRCC metastasizes, it can no longer be resected and requires systemic treatments. Investigators want to see how ccRCC patients respond to combined intravenous infusions of Avelumab and IL-15. IL-15 is a stimulatory protein that produces activation and expansion of immune white blood cells called lymphocytes that are able to attack cancer cells. Avelumab is an anti-PD-L1 antibody that functions as an “immune checkpoint inhibitor” to overcome tumor suppression of immune system. Killer lymphocytes can also uniquely bind to one end of Avelumab that can improve their ability to traffic to sites of tumor. Participants in this study must have ccRCC that has spread to other sites in the body and has not responded to at least two prior therapies with standard licensed treatments for ccRCC.
Clinicaltrials.gov identifier: NCT04150562
NCI Protocol ID: NCI-20-C-0007
Official Title: Avelumab (Bavencio) With IL-15 in Subjects With Clear-cell Renal Carcinoma
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.