Masaki Terabe, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37 Room 1016A
- Bethesda, MD 20892
- 240-760-6731
- terabe@mail.nih.gov
RESEARCH SUMMARY
Dr. Terabe is a trained immunologist who heads the Basic Immunology Research Program at the Laboratory of Integrative Cancer Immunology (LICI). He seeks to understand the mechanisms of immune regulation in cancer patients. He hopes to elucidate what drives the immune system to fail to eliminate a tumor—and use those findings to therapeutically trigger the immune system to act more effectively against the tumor (an approach called immunotherapy). His research currently focuses on natural killer T (NKT) cells, a lesser-known component of the immune system with the response rate of the innate immune system but the antigen specificity of the adaptive immune system. They are unique cells that can be recruited to attack tumors and/or help other immune cells destroy malignant cells. Dr. Terabe hopes to translate his knowledge on NKT cells to new cancer treatments that can help patients combat brain tumors more effectively.
Areas of Expertise

Masaki Terabe, Ph.D.
Research
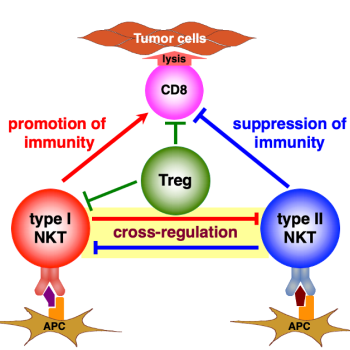
Dr. Terabe has been a passionate immunology researcher for over 20 years, specializing in T-lymphocytes. Since joining the Laboratory of Integrative Cancer Immunology (LICI) to lead the Basic Immunology Research Program, he has focused on determining the role of natural killer T (NKT) cells in the context of brain tumors. While there are various types of T-lymphocytes, NKT cells stand out for a few reasons. First, NKT cells prefer lipids as their antigen of choice, which are enriched or highly expressed in the brain. Second, NKT cells mount a response as quickly as the innate immune system, while retaining the antigen specificity of T-cells in the adaptive immune system. Because tumor immunity is driven by many immune system components that can either decrease or promote growth, NKT cells with specific lipid targets in the brain could be ideal mediators of immunotherapy to suppresses malignancy.
There are several upcoming projects in Dr. Terabe’s lab investigating the role of NKT cells specifically in glioblastoma, which is an area that has not been previously well investigated. Dr. Terabe hopes to elucidate how tumor growth or development—as well as immune cell recruitment to the tumor site—may differ in the absence of NKT cells in mouse models of the immune system. NKT cells have been known to specifically target lipids, which can undergo changes in expression upon tumor development and progression in various cancers.
Another focus of the lab is sulfatide, a lipid in the myelin sheath that can be recognized by NKT cells. Sulfatide could assist in recruiting NKT cells to the tumor, even if the lipids undergo minor changes in the tumor microenvironment.
Finally, because NKT cells have an innate response component and are often referred to as innate T-lymphocytes, Dr. Terabe’s team hopes to elucidate the mechanisms behind tumor immunity in glioblastoma with a specific focus on NKT cells.
Publications
- Bibliography Link
- View Dr. Terabe's Complete Bibliography at NCBI
Targeting the CD47/thrombospondin-1 signaling axis regulates immune cell bioenergetics in the tumor microenvironment to potentiate antitumor immune response
MAIT cells and MR1 play an immunosuppressive role in glioblastoma through the induction of neutrophils and MDSCs
Mouse nEoanTigen pRedictOr pipeline (METRO)
The Role of NKT Cells in Glioblastoma
Unique challenges for glioblastoma immunotherapy-discussions across neuro-oncology and non-neuro-oncology experts in cancer immunology. Meeting Report from the 2019 SNO Immuno-Oncology Think Tank.
Biography

Masaki Terabe, Ph.D.
Dr. Terabe joined the Laboratory of Integrative Cancer Immunology (LICI) to lead the Basic Immunology Research Program and extend his immunology expertise to brain tumors. He received his Ph.D. from the University of Tokyo in 1999, studying immune responses to parasitic infections. He subsequently arrived at the NCI as a visiting fellow at the Molecular Immunogenetics and Vaccine Section of the Metabolism Branch, which is now part of the Vaccine Branch. He later graduated to research fellow (2002-2005), staff scientist (2005-2007), associate scientist (2007-2017), and senior associate scientist (2017-2019). He began collaborating with investigators on tumor immunology projects, and gained insight into how studying the immune system can aid treatment interventions. Dr. Terabe joined the NOB as an investigator in early 2019 in order to pursue this long-standing interest in brain tumors—and investigate the role of immunology in glioblastoma.
In addition to his impressive research career in immunology, Dr. Terabe has published over eighty papers and received numerous awards. Notably, he was awarded the NCI Director’s Innovation Award in 2006 and again in 2010, along with the NCI Technology Transfer Award thirteen times over the last eighteen years. He currently has 16 patents either in process or already issued. He also serves as a board member for The Open Cancer Immunology Journal, while also serving as a reviewer and editor for numerous other journals.
Honors, Awards and Leadership
- Technology Transfer Award, Center for Cancer Research, National Cancer Institute (consecutive years ranging from 2001, 2003-2012, 2014-2016)
- Janson Award, Alumni Association of Veterinary Medicine and Animal Science, University of Tokyo (2011)
- Director’s Innovation Award, National Cancer Institute (2006, 2010)
- AAI Junior Faculty Award, American Association for Immunologists (2004)
- Special Act or Service Cash Award, National Cancer Institute (2004, 2017)
- The Fellows Award for Research Excellence, National Institutes for Health (2001, 2003)
- Postdoctoral Fellow Award - Best Paper of the Year, The NIH Cytokine Interested Group (2001)
Societies and Initiatives
- American Association of Immunologists (AAI)
- American Association of Cancer Research (AACR)
- Japanese Society for Immunology (JSI)
- Society for Immunotherapy of Cancer (SITC)
- Society for Neuro-Oncology (SNO)
- Board member, The Open Cancer Immunology Journal (2008- 2013)
- Review Editor, Frontiers in Antigen Presenting Cell Biology (2010 - present) and Frontiers in Oncology (2019 - present)
- Grant Reviewer, The Cancer Society of New Zealand (2011), Austrian Academy of Science (2014), Czech Science Foundation (2015), and Cancer Research UK (2015)
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
Immunology and the Brain: Scientific Discoveries Needs to Improve Patient Outcomes
February 9, 2022
As the lead of the Basic Immunology Research Program, Dr. Terabe investigates the functions of T cells in brain and spine cancers, in order to glean a better understanding of glioblastoma immunobiology. Read more >
Neuro-Oncology Branch 2020 Year in Review
January 22, 2021
Together, our clinical team, research laboratories, and staff have made meaningful advancements to improve outcomes for patients and their families impacted by brain and spine tumors. Read more >
Celebrating National Mentoring Month
January 31, 2020
We celebrate our impactful mentors in the Neuro-Oncology Branch for National Mentoring Month. Our postdoctoral fellows share their love for science and how their mentors have made their experience at NIH more rewarding. Read more >