Brad St. Croix, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 560, Room 32-34
- Frederick, MD 21702-1201
- 301-846-7456
- stcroixb@mail.nih.gov
RESEARCH SUMMARY
Dr. St. Croix pioneered the development of methods to uncover molecules expressed on the tumor vasculature. He is now exploiting this new knowledge to better understand the process of new blood vessel formation, i.e. angiogenesis, and target the neovasculature that feeds tumors in order to develop more effective therapies for cancer. His recent studies describe the development of new therapeutic monoclonal antibodies that are non-toxic and have potential to aid in the management of cancer and other vascular diseases. The Tumor Angiogenesis Unit develops new approaches to detect and treat primary and metastatic disease through targeting of the tumor microenvironment.
Areas of Expertise

Brad St. Croix, Ph.D.
Research
Tumor Angiogenesis Unit
New blood vessel formation, or angiogenesis, is a critical hallmark of solid tumor growth and anti-angiogenic agents have become a vital component of current cancer treatment regimens. The appeal of anti-angiogenic therapy can be attributed to several advantages of targeting the endothelial cells that line blood vessels, rather than the tumor cells themselves. First, endothelial cells are directly exposed to circulating blood, facilitating drug delivery and enabling the use of high molecular weight therapeutics. Second, each vessel capillary supports hundreds of tumor cells. Third, endothelial cells are genetically stable and their ability to develop resistance may be limited. Finally, this type of therapy should be applicable to a wide variety of tumor types. Several anti-angiogenic agents that target the vascular endothelial growth factor (VEGF) pathway have been approved for the treatment of cancer. However, tumors can exploit alternative angiogenesis mechanisms when the VEGF pathway is blocked.
A major current goal of the Tumor Angiogenesis Unit is to understand the mechanisms of VEGF-independent angiogenesis that can lead to persistent tumor angiogenesis and tumor growth. We are also working to understand the biological functions of several cell surface Tumor Endothelial Markers (TEMs) that were previously found to be elevated in the angiogenic vessels of tumors. Two receptors, TEM5/GPR124 and TEM8/ANTXR1, are of particular interest because of their critical role in angiogenesis. We are using several genetically engineered mouse models to understand the functional role of these TEMs in vivo. We are also developing novel therapeutics, such as TEM8 neutralizing antibodies, in order to block angiogenesis and tumor growth. The end goal of this research is to use new molecular information on tumor angiogenesis to develop clinically useful agents for improved diagnostics and therapeutics of cancer and other vascular diseases.
Publications
Cancer cell survival depends on collagen uptake into tumor-associated stroma
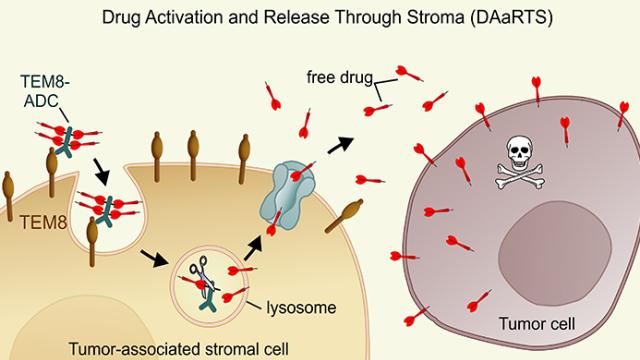
Tumor stroma-targeted antibody-drug conjugate triggers localized anticancer drug release.
Biography

Brad St. Croix, Ph.D.
Dr. Brad St. Croix received his Ph.D. in Medical Biophysics from the University of Toronto in 1998, under Dr. Robert S. Kerbel. He trained as a postdoctoral fellow in the laboratory of Dr. Bert Vogelstein at Johns Hopkins University under a fellowship from the National Cancer Institute of Canada. He joined the Mouse Cancer Genetics Program in 2002. His research focuses on the identification of molecules involved in human tumor angiogenesis and utilizes mouse models to translate new molecular information on angiogenesis into the development of novel diagnostics and therapeutics for cancer.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
Congratulations to Yang for her 2023 Cell Reports: paper!
The NCI Poster: Enhanced Antibody-Drug Conjugate Eradicates Large Breast Cancer Tumors in Experimental Models
ScienceFeatured: New Precision Cancer Therapy Targets Hard-to-Treat Tumors
Congratulations to Frank for his 2022 Nat Comm: paper!
CCR News headlines: Researchers exploit novel vulnerability to deprive solid tumors of nutrients.
Congratulations to Chris for his 2018 J Clin Invest: paper!
McCann JV, Null JL, & Dudley AC, J Clin Invest: Deadly DAaRTS destroy cancer cells via a tumor microenvironment-mediated trigger
CCR News headlines: Antibody-linked drug shrinks various types of tumors in preclinical study
Congratulations to Steve for his 2017 Cancer Cell paper!
Khan KA & Kerbel RS, Cancer Cell: A CD276 Antibody Guided Missile with One Warhead and Two Targets: The Tumor and Its Vasculature.
CCR News headlines: Antibody-linked drug destroys tumor cells and tumor blood vessels in many types of cancer
Cancer Discovery: An Anti-CD276 Antibody–Drug Conjugate Kills Tumor Cells and Vasculature
The Dossier newsletter: September 2017, Issue 29: The Authors Corner.
Congratulations to Lihong for her 2014 Science Translational Medicine paper!
Cancer Discovery: Dual COX2–VEGF Blockade Suppresses Tumor Angiogenesis and Metastasis
Insite: Arthritis Drugs Might Enhance Cancer Chemotherapy
Congratulations to Mi Young for her Gpr116 paper that made the May 2013 cover of Cell Reports!
Congratulations to Amit for his 2012 Cancer Cell paper!
Bettegowda C, Science Translational Medicine: Staying Alive: Specifically Targeting Tumor Vessels with Anti-Angiogenesis Therapy
Cain C. Science-Business eXchange: A TEM to kill
Congratulations to Steve for his 2007 Cancer Cell paper!
Li JL, Harris AL, Cancer Cell: The potential of new tumor endothelium-specific markers for the development of antivascular therapy
Robey, R. Nature Reviews Cancer: Angiogenesis: Hidden signatures written in blood
NIH News Release: NCI Researchers Discover Genes That Are Turned On at High Levels in Tumor-Associated Blood Vessels of Mice and Humans
Cell Press Science News: Tumor vessels identified by unique molecular markers
NIH Research Matters: Genes Turned on in Tumor-Associated Blood Vessels