Christopher J. Westlake, Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 560, Room 22-96C
- Frederick, MD 21702-1201
- 301-846-7594
- 301-846-1666
- westlakecj@mail.nih.gov
RESEARCH SUMMARY
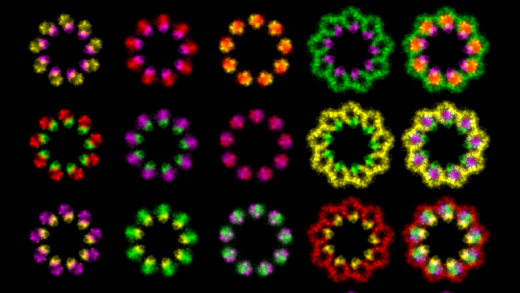
Dr. Westlake’s laboratory uses advanced fluorescence and electron microscopy imaging techniques, biochemical approaches and zebrafish models to investigate membrane trafficking processes regulated by the Rab small GTPase family that are important in development and disease. His current work focuses on a Rab11-Rab8 cascade which functions in primary cilium formation and apical membrane formation in polarized cells. His lab is also investigating the role of Rabs in the regulation of ciliary Hedgehog signaling. Defects in primary cilium formation and function are important in ciliopathy, diseases linked to primary cilia dysfunction, and cancer.
Areas of Expertise

Christopher J. Westlake, Ph.D.
Research
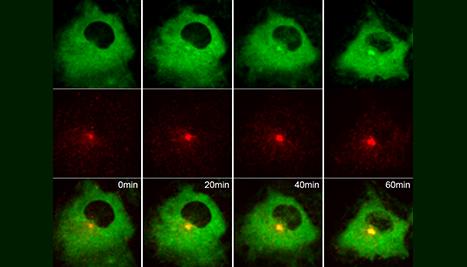
My laboratory studies regulation of cellular membrane transport critical in development and normal homeostasis. Our focus has been on understanding the role of membrane trafficking regulators in ciliogenesis and ciliary signaling, including the Hedgehog pathway. Specifically, we are investigating the role of the Rab small GTPase family and associated trafficking regulators in the transport of membranes and signaling cargo at the cilium.
Using cell and zebrafish models along with cellular imaging, biochemistry and proteomics, and genetics systems we have discovered mechanisms for how ciliogenesis is initiated, characterized new regulators of cilium assembly, identified novel ciliary trafficking pathways, and connected these processes to human disorders and cancer. Our findings also provide insight into the basic biological processes of membrane trafficking that are affected in a wide range of human disease states.
For our cellular imaging studies, we utilize cutting-edge approaches including; live-cell and super resolution microscopy (SIM, STED, STORM), transmission electron microscopy, focused ion beam scanning electron microscopy (FIB-SEM), and correlative light and electron microscopy (CLEM).
Postdoctoral Fellowships
The Membrane Trafficking and Signaling Section has an opening for an outstanding postdoctoral fellow and staff scientist to explore membrane trafficking function in organelle biogenesis and signaling using advanced cellular imaging approaches. Please send your C.V. and statement of interest to chris.westlake@nih.gov
Publications
Variants in the WDR44 WD40-repeat domain cause a spectrum of ciliopathy by impairing ciliogenesis initiation
Akt Regulates a Rab11-Effector Switch Required for Ciliogenesis
Investigation of F-BAR domain PACSIN proteins uncovers membrane tubulation function in cilia assembly and transport
Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation
Biography

Christopher J. Westlake, Ph.D.
In 2004, Dr. Westlake obtained his Ph.D. in Biochemistry under the supervision of Dr. Roger Deeley at Queen's University in Canada. While there, he investigated the structure/function and trafficking of multidrug resistance proteins (MRP) that are important in resistance to chemotherapeutics. He then joined Dr. Richard Scheller at Genentech as a postdoctoral fellow investigating membrane trafficking regulation by the Rab small GTPase family. In 2011, Dr. Westlake joined the Laboratory of Cell and Developmental Signaling at the Center for Cancer Research where he studies membrane trafficking pathways important in ciliopathy, diseases linked to primary cilia dysfunction, and cancer. He was awarded tenure from NIH in 2020.
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
NEWS
CCR News, June 13, 2019
Akt protein kinase pathway regulates key step in the initiation of cilia formation