Jing Wu, M.D., Ph.D.
- Center for Cancer Research
- National Cancer Institute
- Building 37, Room 1142A
- Bethesda, MD 20892
- 240-760-6036
- 301-480-5124
- jing.wu3@nih.gov
RESEARCH SUMMARY
Dr. Wu is a clinical neuro-oncologist who leads the Translational Research Program at the Neuro-Oncology Branch (NOB). Her clinical interests revolve around understanding challenges in neuro-oncology care, and developing laboratory and clinical research programs to understand glioma biology and investigate novel therapeutic approaches to improve patient clinical outcomes. Her goal is to observe phenotypes and gaps in knowledge through her clinical practice, in order to address these questions in the laboratory and subsequently develop hypothesis-based clinical trials.
There are two facets to Dr. Wu’s research. First, she seeks to understand gliomas that exhibit an aggressive phenotype, in order to develop a diagnostic tool to monitor their transformation from low-grade to high-grade disease when they exhibit rapid growth. Her second goal is to test the efficacy of the multi-kinase inhibitor TG02 in clinical trials, in order to understand its effects on high-grade astrocytomas and IDH-mutant gliomas.
Explore the NOB's Research Programs >
Areas of Expertise
Information for Patients
Learn about our multidisciplinary approach to patient care, our clinical trials and the highly specialized care teams who lead them.

Jing Wu, M.D., Ph.D.
Clinical Trials
Research
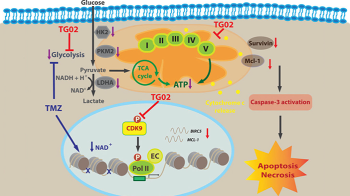
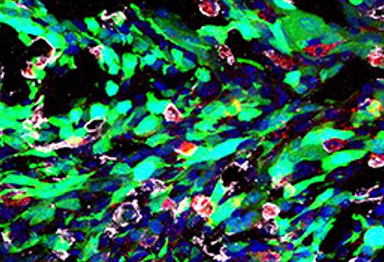
disruption, the cells undergo apoptosis and necrosis.
Credit: Courtesy of Dr. Wu
Dr. Wu’s Translational Research Program is focused on the clinical aspects of gliomas, which are rarely fully curable—whether or not they’re diagnosed as low- or high-grade tumors. Gliomas also exhibit high levels of heterogeneity, making them challenging to treat long-term. Isocitrate dehydrogenase (IDH) genes are mutated in approximately five percent of all gliomas and eighty percent of low-grade gliomas, causing 2-hydroxyglutarate (2-HG) to accumulate and leading to increased tumorigenic potential. While individuals with IDH-mutant low-grade gliomas often have slower disease progression and respond more favorably to treatment than those with IDH wildtype tumors, almost all cases eventually transform into more aggressive high-grade gliomas that rapidly progress.
As there are significant gaps in knowledge regarding how and why this transformation to high-grade disease occurs, the first facet of Dr. Wu’s research focuses on developing a diagnostic tool to detect this transformation as early as possible in patients. Levels of 2-HG and/or metabolic flux may change as high-grade transformation takes place. This is because glioma cells must undergo a metabolic shift to aerobic glycolysis, in order to support their rapid growth. The level of 2-HG can be detected using 1H based magnetic resonance spectroscopic imaging (MRSI), and the metabolic flux can be detected by administering a 13C hyperpolarized pyruvate MRSI noninvasively into the patient—resulting in real-time tracking of the tumor’s changes. These techniques will help uncover the intracellular changes that indicate tumor progression without the need for biopsy or surgery.
Once gliomas transform to high-grade status, those with increased mutational burden may respond better to immunotherapy. One key feature of high-grade gliomas is the enormous intra- and inter-tumoral heterogeneity that drives their aggressiveness and therapy resistance. To combat this, Dr. Wu’s second project elucidates whether TG02, a multi-kinase inhibitor, primarily inhibits cyclin-dependent kinase 9 (CDK9) and can target multiple cancer survival pathways simultaneously. This agent can cross the blood-brain barrier, making it a favorable candidate for glioma treatment. It has also shown preclinical benefit when combined with Temozolomide, a common alkylating chemotherapeutic agent in brain tumor treatment. This research has led to an actively recruiting phase 1/2 clinical trial for patients with recurrent high-grade gliomas.
Publications
Phase I Study of Zotiraciclib in Combination with Temozolomide for Patients with Recurrent High-grade Astrocytomas.
A Bayesian Adaptive Randomized Phase II Multicenter Trial of Bevacizumab with or without Vorinostat in Adults with Recurrent Glioblastoma.
MerTK inhibition decreases immune suppressive glioblastoma-associated macrophages and neoangiogenesis in glioblastoma microenvironment.
Novel Targeting of Transcription and Metabolism in Glioblastoma
Chemosensitivity of IDH1-Mutated Gliomas Due to an Impairment in PARP1-Mediated DNA Repair
Biography

Jing Wu, M.D., Ph.D.
Dr. Wu received her Doctor of Medicine degree from Capital Medical University in Beijing, China prior to moving to the United States and pursuing her Ph.D. in neuroscience from the University of Texas Medical Branch at Galveston. She subsequently completed an NIH postdoctoral fellowship under William D. Willis, who was a significant contributor to the fields of pain and neuroscience and a lifelong mentor to Dr. Wu. Following her Ph.D., she completed an internship in internal medicine at Texas Tech University Medical Center, followed by a neurology residency at The University of Texas Health Science Center (where she also served as the chief resident), and a Neuro-Oncology fellowship at The University of Texas MD Anderson Cancer Center. She then joined the University of North Carolina (UNC) at Chapel Hill as a tenure-track assistant professor in the Department of Neurosurgery and Neurology. She served as the co-director of the Brain Tumor Program at the Lineberger Comprehensive Cancer Center and developed a clinical and translational research program in neuro-oncology at UNC. Dr. Wu joined the Neuro-Oncology Branch (NOB) in 2015 as a staff physician, and soon became the director of the Neuro-Oncology Fellowship Program as well as a tenure-track investigator overseeing multiple clinical trials.
Dr. Wu is certified both by the American Board of Psychiatry and Neurology (ABPN) in neurology and the United Council for Neurologic Subspecialties (UCNS) in neuro-oncology. She has published over 50 peer-reviewed articles while also serving as an invited reviewer for several prestigious journals. She has received impressive awards over her training and clinical tenure, including the William James Miller Endowed Fellowship Award in Neuro-Oncology and the NIH/NCI Paul Calabresi Clinical Oncology Scholar Award. In 2018, she was awarded the NIH-Lasker Clinical Research Scholar Award—a prestigious opportunity that provides funding and support for exceptional clinical researchers to develop their clinical research programs.
Honors, Awards and Leadership
- NCI Director’s Award for Translational Science - 2022
- Excellence in Technology Transfer Award for “Zotiraciclib, US FDA and EMA Orphan Drug Designation for Glioma,” Federal Laboratory Consortium for Technology Transfer - 2020
- NIH-Lasker Clinical Research Scholar - 2018
- NIH/NCI Paul Calabresi Clinical Oncology Scholar Award (K12 Career Award) - 2011-2014
- Junior Faculty Scholarship for “Translational and Clinical Research Course for Clinician-Scientist,” American Neurological Association - 2011
- William J. Miller Endowed Fellowship Award in Neuro-Oncology, MD Anderson Cancer Center - 2009
- Frank M. Yatsu MD Excellence in Residency Award, University of Texas Health Science Center - 2008
- Teva Neuroscience Excellence in Teaching Award, University of Texas Health Science Center - 2008
- Residents’ Scholarship Award, American Academy of Neurology - 2006
- Jeanne B. Kempner Scholar Award, Jeanne B. Kempner Research Foundation - 2000-2001
- Curtis W. Lambert Scholarship Award, University of Texas - 1999
- George Sealy Excellence Research Award in Neurology, University of Texas - 1998
- Anatomy and Neuroscience Research Award (39th National Student Research Forum) - 1998
Societies and Initiatives
- NCI Brain Malignancies Steering Committee (BMSC), The Coordinating Center for Clinical Trials (CCCT), NCI, NIH - 2022-present
- NRG Oncology Group - 2021-present
- Center for Cancer Research (CCR) Clinical Review Panel, NCI, NIH - 2020-present
- Center for Cancer Research (CCR) Science Board Member, NCI, NIH - 2016-present
- Collaborative Ependymoma Research Network (CERN) - 2011-present
- Brain Tumor Trials Collaboratives Consortium (BTTC) - 2011-present
- Society for Neuro-Oncology (SNO) - 2008-present
- Co-Chair, Oligodendroglioma Workshop, CCR, NIH - 2018
- The Alliance for Clinical Trials in Oncology - 2011-2015
- American Society of Clinical Oncology (ASCO) - 2008-2015
- American Academy of Neurology (AAN) - 2006-2015
- Society for Neuroscience - 2000-2010
Job Vacancies
We have no open positions in our group at this time, please check back later.
To see all available positions at CCR, take a look at our Careers page. You can also subscribe to receive CCR's latest job and training opportunities in your inbox.
Team
News
New Clinical Trial Tests a Kind of Precision Medicine Treatment for IDH-Mutant Brain Tumors
February 12, 2024
A new trial investigates zotiraciclib as a treatment for people with recurrent gliomas containing an IDH1 or IDH2 mutation to control tumor growth and improve quality of life. Read more >
FDA grants orphan drug designation to indotecan for the treatment of glioma
November 29, 2023
The FDA granted orphan drug status to LMP400 (indotecan) for use in patients with malignant glioma, a cancer of the brain that begins in glial cells. Read more >
Powerful Drug Combination Kills Glioblastoma Tumors Containing a Unique Genetic Makeup
September 19, 2023
Researchers conducted preclinical experiments to explore a treatment that damages brain tumors’ DNA and prevents the cells from repairing it. Read more >
Clinical Trial Researches Drug Therapy for CNS Tumors
May 5, 2023
A clinical trial led by Dr. Jing Wu is researching a drug therapy for gliomas that contain mutations in IDH1 and IDH2 genes, which are especially aggressive. Read more >
July 20, 2023
As head of the Translational Research Program, Dr. Jing Wu aims to better understand gliomas and identify potential therapies to treat these tricky brain tumors. Read more >
Neuro-Oncology Branch Members Earn NCI Director’s Awards for Improving Patient Care and Outcomes
April 5, 2023
Dr. Terri Armstrong, Alvina Acquaye-Mallory, and Tracy Ani were recognized for their efforts to make clinical trials more inclusive, while Dr. Jing Wu was recognized for her research on the anticancer drug zotiraciclib. Read more >
Translational Research: Turning Patient Observations and Laboratory Findings into Clinical Trials
September 28, 2021
The Translational Research Program, led by Dr. Jing Wu, leverages laboratory research and patient observations to find new ways to treat brain and spine tumors, as well as improve patient outcomes. Read more >
Women Scientist Appreciation Month
March 1, 2020
In honor of International Women and Girls in Science Day, we celebrated female scientists, physicians and mentors in the NOB that strive every day to make advances in ground-breaking research for brain and spine tumor patients. Read more >
FDA Grants Orphan Drug Designation to Zotiraciclib for the Treatment of Glioma
January 9, 2020
Dr. Jing Wu's investigational drug, zotiraciclib, received orphan drug approval by the FDA for the treatment of glioma, which comprises up to 30% of all brain and central nervous system tumors. Read more >