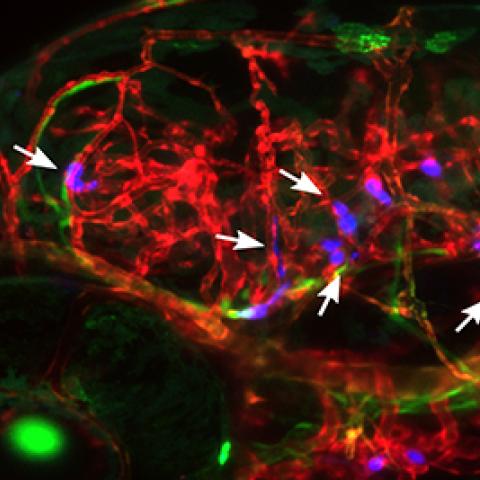
Human breast tumor cells arrested in the blood vessels of zebrafish larvae, five days after the tumor cells were injected into the zebrafish circulation. The vasculature is displayed in red, the venous circulation in green and injected cancer cells in blue.
Photo courtesy of Kandice Tanner
In CCR’s Laboratory of Cell Biology, Kandice Tanner, Ph.D., and her team are watching human cancer cells travel through the bodies of small, translucent fish. As the cells make their way through the animals’ circulatory systems to reach organs where they can establish themselves and begin to grow, the researchers are learning about the factors that drive cancer’s spread through the body.
Tanner, postdoctoral fellow Colin Paul, Ph.D., and colleagues report in the August 21, 2019, issue of Cell Systems that they have used zebrafish to discover that the particular sites colonized by migrating tumor cells are determined by both the physical architecture of tissue and the cells’ molecular compatibility with their microenvironment.
Metastasis, the spread of cancer away from a primary tumor to new locations in the body, is an unpredictable and potentially lethal process. It is not random: Cancers of the same type tend to metastasize to certain locations. When breast cancer spreads, for example, it most often makes its way to a patient’s bones, brain, liver or lungs. It’s not known, however, to what extent tumor cells are programmed to seek out particular sites, and currently there is no way of knowing where an individual patient is most likely to develop metastatic lesions.
Dr. Tanner is particularly interested in how cells sense, respond and alter their physical surroundings. She and her colleagues turned to zebrafish to study the early events in cancer metastasis because the tiny, transparent bodies of living zebrafish larvae can be viewed in their entirety under a microscope, making it possible to follow the movements of individual cells wherever they go. “The zebrafish is a powerful tool because we can follow the same cells in the same animal over several days,” she says. “We can get quantitative data to really understand what drives non-random organ targeting.”
Working with Raman Sood, Ph.D., who heads the Zebrafish Core at NIH’s National Human Genome Research Institute, Tanner’s team injected human cancer cells into the circulatory systems of developing fish when the animals were just a few millimeters long. Over the next five days, they monitored the cells as they moved through the circulatory system and some made their way into either the brain or a structure called the caudal vascular plexus (CVP), an environment that Tanner says is functionally similar to bone marrow. The cells the scientists used for their experiments tend to develop into tumors in the brain and bone marrow in mice as well, suggesting they behave similarly in the fish and in mammals.
Physically, Tanner says, blood vessels in the brain are different from those in the CVP. In the brain, tumor cells travel through straight and narrow vessels, whereas in the CVP, they follow a more variable, twisted path. The researchers found that migrating tumor cells were more likely to stop their journey amidst this relative chaos than they were to become arrested in the brain. Once the tumor cells’ movement had stalled, some managed to work their way out of the blood vessels into surrounding tissue. The team showed that this step, which is essential for metastatic tumor cells to begin to colonize an organ, was dependent on a cell’s molecular makeup with certain cells being better suited to invade the CVP and others establishing themselves more readily in the brain.
Tanner and her colleagues found that by genetically manipulating the tumor cells that preferred the brain, they could make those cells go to the CVP instead. With a deeper understanding of the factors that drive the early events in metastasis, it might one day be possible to redirect circulating metastatic tumor cells in patients at the time their primary tumors are treated, she says. Such an intervention could ensure that if new tumors arise, they will be more likely to occur in sites where they will be less harmful or easier to treat.