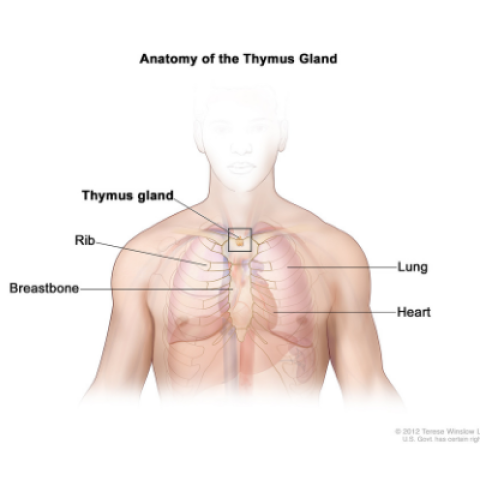
Anatomy of the thymus gland; drawing shows the thymus gland in the upper chest under the breastbone. Also shown are the ribs, lungs, and heart.
Image Source: NCI Visuals Online
Adults whose thymoma or thymic carcinoma has progressed after treatment with at least one platinum-containing chemotherapy drug may be eligible to participate in a clinical trial at the NIH Clinical Center.
Arun Rajan, M.D., Senior Clinician in the Thoracic and GI Malignancies Branch, is leading a clinical trial of a treatment for thymoma and thymic carcinoma that has returned after chemotherapy and cannot be treated with surgery. Thymic epithelial tumors (TETs) are rare cancers that originate from the cells of the thymus, a small organ that sits in the chest between the lungs and above the heart. The growth potential of cancers of the thymus can vary. Thymomas generally demonstrate slower growth, whereas thymic carcinomas can grow rapidly. However, all TETs have the ability to spread widely to various organs and become difficult to treat. There are no approved drugs for treating TETs that have come back after chemotherapy. Researchers want to see if combination immunotherapy with the drug Bintrafusp alfa (M7824) can help patients with recurrent TETs. Bintrafusp alfa blocks two immunological pathways that are potentially involved in helping cancer cells escape attack by the immune system. Once these pathways are blocked, cells of the immune system have an improved chance of killing cancer cells. Bintrafusp alfa has not been studied as a treatment for TETs, but in studies in other types of cancer it has shown promising results with fewer side effects than chemotherapy.
Clinicaltrials.gov identifier: NCT04417660
NCI Protocol ID: NCI-20-C-0097
Official Title: A Phase II, Open-Label Trial of Bintrafusp Alfa (M7824) in Subjects With Thymoma and Thymic Carcinoma
The Center for Cancer Research is NCI’s internal cancer center, a publicly funded organization working to improve the lives of cancer patients by solving important, challenging and neglected problems in cancer research and patient care. Highly trained physician-scientists develop and carry out clinical trials to create the medicines of tomorrow treating patients at the world’s largest dedicated research hospital on the campus of the National Institutes of Health in Bethesda, Maryland.
For more information on CCR clinical trials click here, and subscribe to have the latest CCR clinical trials sent directly to your inbox.