News and Events
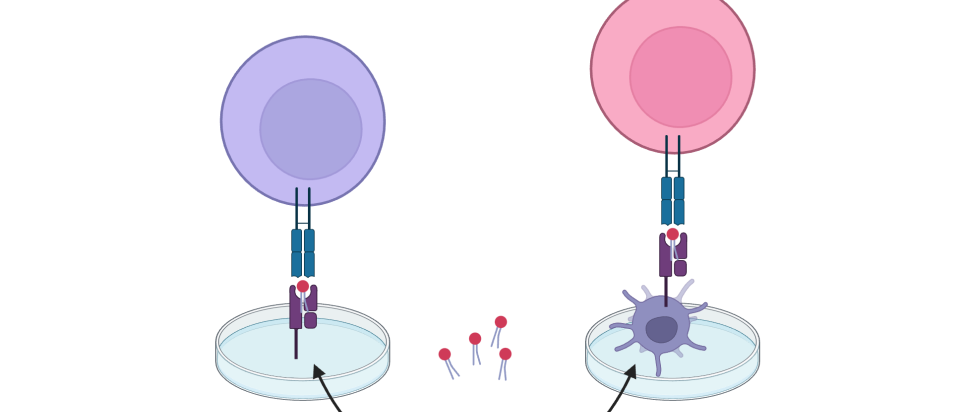
Cellular processing reverses molecule’s effect on anticancer immunity
Immune cells convert an immunosuppressive lipid into an anticancer immunity enhancer.
Read MoreNIH researchers develop AI tool with potential to more precisely match cancer drugs to patients
In a proof-of-concept study published on April 18, 2024, in Nature Cancer, CCR researchers have developed an artificial intelligence (AI) tool that uses data from individual cells inside tumors to predict whether a person’s cancer will respond to a specific drug. The team, led by Eytan Ruppin, M.D., Ph.D., Chief of the Cancer Data Science Laboratory, suggests that such single-cell RNA sequencing data could one day be used to help doctors more precisely match cancer patients with drugs that will be effective in treating their cancer.
Read MoreJung-Min Lee appointed Senior Investigator at CCR
The CCR community congratulates Jung-Min Lee, M.D., who has been appointed as a Senior Investigator in the Women’s Malignancies Branch. Lee’s research focuses on developing targeted therapies for ovarian carcinoma. Her studies have identified key proteins of DNA damage response pathways as therapeutic targets, opening up possibilities for novel therapies for this disease. Her research also emphasizes the collection of patient samples to better understand treatment response and tumor biology.
Read MoreAligned Blog: Honoring Women's History
As Women's History Month comes to an end, Shauna Clark, Ph.D., Associate Director of CCR's Office of Equity and Inclusion, highlights some of the work being done at the NIH in support of the inclusion of women in research and clinical trials.
Read MoreClinical trial researching medication therapy for IDH2-mutated nasal cavity or skull base tumors
A clinical trial led by Charalampos S. Floudas, M.D., D.M.Sc., M.S., Assistant Research Physician in the Center for Immuno-Oncology, is researching targeted medication therapy for patients with IDH2-mutated tumors in the nasal cavity or skull base area.
Read MoreLeah Cook appointed Senior Investigator at CCR
The CCR community welcomes Leah M. Cook, Ph.D., who has been appointed as a Senior Investigator in the Cancer Innovation Laboratory. She is a cancer biologist with a focus on metastasis and the metastasis microenvironment. The goal of her research program is to identify mechanisms associated with bone metastatic prostate cancer and specifically, the underpinnings of the immune-tumor bone environment that contribute to metastatic disease.
Read MoreClinical trial researching post-treatment care for prostate cancer
A trial led by Deborah E. Citrin, M.D., Senior Investigator in the Radiation Oncology Branch, is studying the use of a device for improving urinary issues after prostate cancer treatment.
Read MoreFirst Cancer TIL Therapy Gets FDA Approval for Advanced Melanoma
On February 16, 2024, the Food and Drug Administration approved lifileucel (Amtagvi), the first cancer treatment that uses immune cells called tumor-infiltrating lymphocytes (TILs), for some people with advanced melanoma. TIL therapy was pioneered in the 1980s by Steven A. Rosenberg, M.D., Chief of the Surgery Branch, and the final approval of this treatment has taken years of clinical trial research and ongoing partnerships among multiple institutions.
Read MoreClinical trial researching prostate cancer in African American adults
Ismail Baris Turkbey, M.D., Senior Clinician in the Molecular Imaging Branch, is leading a clinical trial to study and improve diagnostic outcomes for prostate cancer in African American adults.
Read MoreIn Memoriam: C. Norman Coleman, M.D.
The CCR community is deeply saddened by the recent passing of C. Norman Coleman, M.D., Senior Investigator in the Radiation Oncology Branch.
Read MoreNew Milestones publication now available
Every year, CCR makes remarkable contributions to the understanding, detection, treatment and prevention of cancer. This issue of our annual publication, Milestones, features 10 of our top scientific advances from the past year. These discoveries fall everywhere on the spectrum from basic science to clinical research, ranging from a change in our understanding of how cells replicate and divide to the first FDA-approved treatment for a rare cancer based on the results of an NCI trial. Our researchers have developed a novel drug delivery system inspired by bacterial spores, identified prognostic gene signatures for patients with different cancers and gathered data over 30 years to show that an immunotherapy essentially cures a rare precancerous disease.
Read More